当前位置:
X-MOL 学术
›
Appl. Organomet. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nanocomposite‐based inorganic‐organocatalyst Cu(II) complex and SiO2‐ and Fe3O4 nanoparticles as low‐cost and efficient catalysts for aniline and 2‐aminopyridine oxidation
Applied Organometallic Chemistry ( IF 3.9 ) Pub Date : 2020-09-07 , DOI: 10.1002/aoc.5999 Mohamed Shaker S. Adam 1, 2 , Mohammed A. Al‐Omair 1
Applied Organometallic Chemistry ( IF 3.9 ) Pub Date : 2020-09-07 , DOI: 10.1002/aoc.5999 Mohamed Shaker S. Adam 1, 2 , Mohammed A. Al‐Omair 1
Affiliation
|
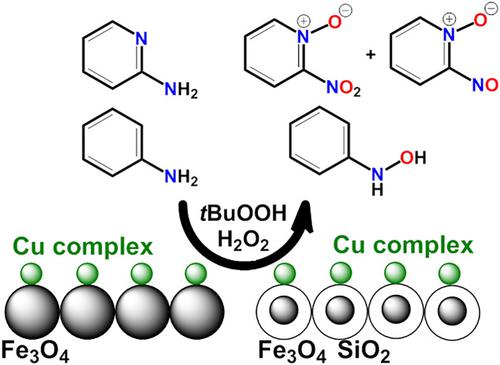
Bis‐imino Cu(II) complex (CuLAn2), in which the imine ligand (HLAn) acts as a bidentate chelating ligand, was synthesized. The catalytic potential of the inorganic‐organocatalyst was studied homogeneously and heterogeneously in the oxidation of aniline and 2‐aminopyridine by H2O2 or tBuOOH. Two heterogeneous inorganic‐organocatalysts, CuLAn2@Fe3O4 and CuLAn2@SiO2@Fe3O4, were synthesized by the successful immobilization of CuLAn2 on the Fe3O4 surface and the composited Fe3O4 with SiO2, respectively. The heterogeneous structure of those inorganic‐organocatalysts was confirmed using Fourier‐transform infrared, scanning electron microscopy, energy‐dispersive X‐ray spectroscopy, X‐ray diffraction, transmission electron microscopy, and magnetic properties. The adsorption–desorption isotherms revealed respectable adsorption parameters (SBET, Vp, and rp). All catalysts exhibited high potential in the oxidation of aniline (with phenylhydroxylamine as the main product) and good potential in the oxidation of 2‐aminopyridine, in the first attempt (with 2‐nitropyridine‐N‐oxide and 2‐nitrosopyridine‐N‐oxide as main products), at room temperature. Acetonitrile was found to be the best solvent compared to ethanol, dimethyl sulfoxide, chloroform, and water. The homogeneous catalyst exhibited reusability for three times. The heterogeneous catalysts, CuLAn2@Fe3O4 and CuLAn2@SiO2@Fe3O4, were active for five and seven times, respectively. A mechanism was proposed within electron and oxygen transfer processes.
中文翻译:

基于纳米复合材料的无机有机催化剂Cu(II)配合物和SiO2和Fe3O4纳米颗粒是苯胺和2-氨基吡啶氧化的低成本高效催化剂
合成了亚氨基配体(HLAn)作为双齿螯合配体的双亚氨基铜(II)配合物(CuLAn 2)。在H 2 O 2或t BuOOH氧化苯胺和2-氨基吡啶的过程中,均质和非均质地研究了无机-有机催化剂的催化潜力。通过成功地将CuLAn 2固定在Fe 3 O 4上,合成了两种非均相无机-有机催化剂CuLAn 2 @Fe 3 O 4和CuLAn 2 @SiO 2 @Fe 3 O 4。表面和分别与SiO 2复合的Fe 3 O 4。通过傅立叶变换红外光谱,扫描电子显微镜,能量色散X射线光谱,X射线衍射,透射电子显微镜和磁学性质证实了这些无机有机催化剂的非均相结构。吸附-解吸等温线揭示了可观的吸附参数(S BET,V p和r p)。所有催化剂在首次尝试中(使用2-硝基吡啶-N)在苯胺(以苯羟胺为主要产物)的氧化中均显示出高电势,在2-氨基吡啶的氧化中显示出良好的电势。-氧化和2-亚硝基吡啶-N-氧化作为主要产物),在室温下。与乙醇,二甲基亚砜,氯仿和水相比,乙腈是最好的溶剂。均相催化剂表现出三倍的可重复使用性。均相催化剂CuLAn 2 @Fe 3 O 4和CuLAn 2 @SiO 2 @Fe 3 O 4分别具有五次和七次活性。在电子和氧气转移过程中提出了一种机制。
更新日期:2020-11-06
中文翻译:

基于纳米复合材料的无机有机催化剂Cu(II)配合物和SiO2和Fe3O4纳米颗粒是苯胺和2-氨基吡啶氧化的低成本高效催化剂
合成了亚氨基配体(HLAn)作为双齿螯合配体的双亚氨基铜(II)配合物(CuLAn 2)。在H 2 O 2或t BuOOH氧化苯胺和2-氨基吡啶的过程中,均质和非均质地研究了无机-有机催化剂的催化潜力。通过成功地将CuLAn 2固定在Fe 3 O 4上,合成了两种非均相无机-有机催化剂CuLAn 2 @Fe 3 O 4和CuLAn 2 @SiO 2 @Fe 3 O 4。表面和分别与SiO 2复合的Fe 3 O 4。通过傅立叶变换红外光谱,扫描电子显微镜,能量色散X射线光谱,X射线衍射,透射电子显微镜和磁学性质证实了这些无机有机催化剂的非均相结构。吸附-解吸等温线揭示了可观的吸附参数(S BET,V p和r p)。所有催化剂在首次尝试中(使用2-硝基吡啶-N)在苯胺(以苯羟胺为主要产物)的氧化中均显示出高电势,在2-氨基吡啶的氧化中显示出良好的电势。-氧化和2-亚硝基吡啶-N-氧化作为主要产物),在室温下。与乙醇,二甲基亚砜,氯仿和水相比,乙腈是最好的溶剂。均相催化剂表现出三倍的可重复使用性。均相催化剂CuLAn 2 @Fe 3 O 4和CuLAn 2 @SiO 2 @Fe 3 O 4分别具有五次和七次活性。在电子和氧气转移过程中提出了一种机制。


























 京公网安备 11010802027423号
京公网安备 11010802027423号