当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
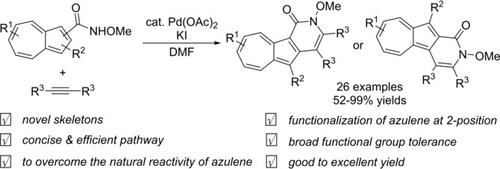
Synthesis of Azulenopyridinones through Palladium‐Catalyzed Oxidative [4+2] Cyclization Reactions of N‐Methoxyazulene‐1‐ and 2‐carboxamides with Alkynes
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-09-07 , DOI: 10.1002/adsc.202000587 Gi Uk Han 1 , Jeong‐Yu Son 1 , Dahee Park 1 , Hyeonsik Eom 1 , Kyungsup Lee 1 , Hee Chan Noh 1 , Kooyeon Lee 2 , Phil Ho Lee 1, 3
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-09-07 , DOI: 10.1002/adsc.202000587 Gi Uk Han 1 , Jeong‐Yu Son 1 , Dahee Park 1 , Hyeonsik Eom 1 , Kyungsup Lee 1 , Hee Chan Noh 1 , Kooyeon Lee 2 , Phil Ho Lee 1, 3
Affiliation
|
Palladium‐catalyzed oxidative [4+2] cyclization reactions were developed from the C−H activation reaction of N‐methoxyazulene‐1‐ and 2‐carboxamides with symmetrical and unsymmetrical alkynes under a molecular oxygen atmosphere, producing azulenopyridinone derivatives with novel azulene skeletons in good to excellent yields with a wide substrate scope and excellent functional group tolerance.
中文翻译:

N-甲氧基azulene-1和2-羧酰胺与炔烃的钯催化氧化[4 + 2]环化反应合成氮杂吡啶并酮
钯催化的氧化[4 + 2]环化反应是由N-甲氧基azulene-1和2-羧酰胺与对称和不对称炔烃在分子氧气氛下的C–H活化反应发展而来的,在此过程中生成具有新颖氮杂环骨架的氮杂吡啶并酮衍生物具有良好的优异产率,具有广泛的底物范围和出色的官能团耐受性。
更新日期:2020-11-04
中文翻译:

N-甲氧基azulene-1和2-羧酰胺与炔烃的钯催化氧化[4 + 2]环化反应合成氮杂吡啶并酮
钯催化的氧化[4 + 2]环化反应是由N-甲氧基azulene-1和2-羧酰胺与对称和不对称炔烃在分子氧气氛下的C–H活化反应发展而来的,在此过程中生成具有新颖氮杂环骨架的氮杂吡啶并酮衍生物具有良好的优异产率,具有广泛的底物范围和出色的官能团耐受性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号