当前位置:
X-MOL 学术
›
Electroanalysis
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemistry of Os Bipyridyl and Phenanthroline Complexes, Comparison with Ru and Fe
Electroanalysis ( IF 3 ) Pub Date : 2020-09-07 , DOI: 10.1002/elan.202060300 Deidré Westhuizen 1 , Jeanet Conradie 1, 2 , Karel G. Eschwege 1
Electroanalysis ( IF 3 ) Pub Date : 2020-09-07 , DOI: 10.1002/elan.202060300 Deidré Westhuizen 1 , Jeanet Conradie 1, 2 , Karel G. Eschwege 1
Affiliation
|
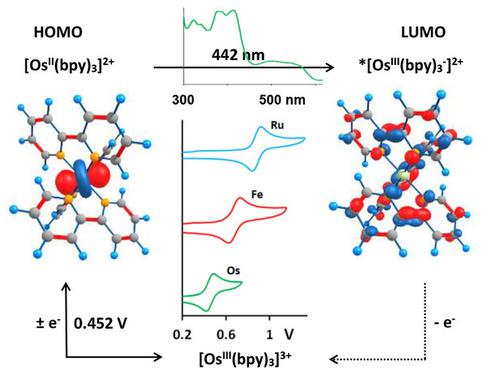
Osmium(II) polypyridyl complexes may be useful in H2O and CO2 photo‐catalytic reduction and dye‐sensitized solar cell research. Altering substituents on the polypyridyl ligands allows for spectral and electronic tuning of the osmium(II) polypyridyl complexes. The cyclic voltammograms and UV‐visible spectra of a series of electronically altered bipyridine and phenanthroline osmium(II) complexes are presented. OsII/III experimental oxidation potentials correlate linearly (R2=0.99) with density functional theory (DFT) implicit solvent model computed ionization potentials and highest occupied molecular orbital (HOMO) energies. The hereby obtained linear relationship provides a theoretical computational tool that may be used for the predetermination of redox potentials of related osmium(II) polypyridyl complexes. Comparison between similar correlations for elements in the same group, namely Fe, Ru and Os, revealed remarkably similar patterns. Of the three, OsII bipyridyl is most readily oxidized (0.452 V), followed by FeII (0.682 V) and then RuII (0.883 V). Spectra of the OsII complexes where λmax values range from 392 to 442 nm, were closely simulated by time‐dependant DFT computed electronic oscillators. Osmium(II) polypyridyl complex absorbances span almost the entire visible spectrum.
中文翻译:

Os联吡啶和菲咯啉配合物的电化学,与Ru和Fe的比较
poly(II)吡啶基络合物可用于H 2 O和CO 2光催化还原以及染料敏化太阳能电池的研究。改变聚吡啶基配体上的取代基可以对spectral(II)聚吡啶基络合物进行光谱和电子调谐。介绍了一系列电子改变的联吡啶和菲咯啉(II)配合物的循环伏安图和紫外可见光谱。Os II / III实验氧化电位线性相关(R 2= 0.99),使用密度泛函理论(DFT)隐式溶剂模型计算电离势和最高占据分子轨道(HOMO)能量。由此获得的线性关系提供了一种理论计算工具,可用于预先确定相关的(II)聚吡啶基配合物的氧化还原电势。同一组中的元素Fe,Ru和Os的相似相关性之间的比较显示出非常相似的模式。在这三种中,Os II联吡啶最容易被氧化(0.452 V),其次是Fe II(0.682 V),然后是Ru II(0.883 V)。Os II络合物的光谱,其中λmax值范围从392 nm到442 nm,是由依赖于时间的DFT计算的电子振荡器严格模拟的。poly(II)吡啶基络合物的吸光度几乎涵盖整个可见光谱。
更新日期:2020-09-07
中文翻译:

Os联吡啶和菲咯啉配合物的电化学,与Ru和Fe的比较
poly(II)吡啶基络合物可用于H 2 O和CO 2光催化还原以及染料敏化太阳能电池的研究。改变聚吡啶基配体上的取代基可以对spectral(II)聚吡啶基络合物进行光谱和电子调谐。介绍了一系列电子改变的联吡啶和菲咯啉(II)配合物的循环伏安图和紫外可见光谱。Os II / III实验氧化电位线性相关(R 2= 0.99),使用密度泛函理论(DFT)隐式溶剂模型计算电离势和最高占据分子轨道(HOMO)能量。由此获得的线性关系提供了一种理论计算工具,可用于预先确定相关的(II)聚吡啶基配合物的氧化还原电势。同一组中的元素Fe,Ru和Os的相似相关性之间的比较显示出非常相似的模式。在这三种中,Os II联吡啶最容易被氧化(0.452 V),其次是Fe II(0.682 V),然后是Ru II(0.883 V)。Os II络合物的光谱,其中λmax值范围从392 nm到442 nm,是由依赖于时间的DFT计算的电子振荡器严格模拟的。poly(II)吡啶基络合物的吸光度几乎涵盖整个可见光谱。


























 京公网安备 11010802027423号
京公网安备 11010802027423号