当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Transition‐Metal‐Free C‐S Bond Forming Strategy towards Synthesis of Highly Diverse Pyrazole Tethered Benzothiazoles: Investigation of their Photophysical Properties
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-09-08 , DOI: 10.1002/ajoc.202000390 Shubham Sharma 1 , Chandi C. Malakar 2 , Virender Singh 1, 3
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-09-08 , DOI: 10.1002/ajoc.202000390 Shubham Sharma 1 , Chandi C. Malakar 2 , Virender Singh 1, 3
Affiliation
|
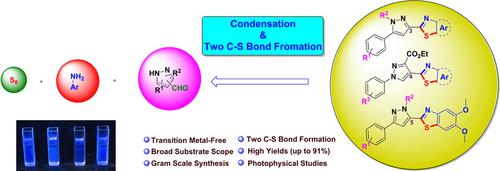
A metal‐free domino approach has been devised towards construction of two C−S bonds for the synthesis of bioactive N, S‐heterocycles. The developed method was extended for the preparation of structurally diverse pyrazole tethered benzothiazole frameworks by using one‐pot operation of pyrazole C‐3/4/5 carbaldehydes, electron rich aromatic amines and elemental sulfur. This protocol provides excellent fluorophores with several additional advantages such as transition metal‐free approach, inexpensive and odorless sulfur source, superior atom economy, and broad substrate scope including gram scale synthesis. The synthesized pyrazole tethered benzothiazole fluorophores were evaluated for their luminescent properties including absorbance, excitation, emission, molar extinction coefficient, brightness, and Stokes shift. The photophysical studies revealed that these pyrazole and benzothiazole hybrids emerged as excellent fluorophores and could exhibit fluorescence quantum yield up to 66%.
中文翻译:

合成高度多样的吡唑系苯并噻唑的无过渡金属CS键形成策略:其光物理性质的研究
已经设计了一种无金属的多米诺方法来构建两个C-S键,以合成具有生物活性的N,S-杂环。通过使用一锅操作吡唑C-3 / 4/5甲醛,富电子芳族胺和元素硫,将开发的方法扩展到制备结构多样的吡唑系留的苯并噻唑骨架。该协议提供了出色的荧光团,并具有其他一些优势,例如无过渡金属的方法,廉价且无味的硫源,优异的原子经济性以及广泛的底物范围,包括克级合成。评价了合成的吡唑束缚的苯并噻唑荧光团的发光性质,包括吸光度,激发,发射,摩尔消光系数,亮度和斯托克斯位移。
更新日期:2020-11-17
中文翻译:

合成高度多样的吡唑系苯并噻唑的无过渡金属CS键形成策略:其光物理性质的研究
已经设计了一种无金属的多米诺方法来构建两个C-S键,以合成具有生物活性的N,S-杂环。通过使用一锅操作吡唑C-3 / 4/5甲醛,富电子芳族胺和元素硫,将开发的方法扩展到制备结构多样的吡唑系留的苯并噻唑骨架。该协议提供了出色的荧光团,并具有其他一些优势,例如无过渡金属的方法,廉价且无味的硫源,优异的原子经济性以及广泛的底物范围,包括克级合成。评价了合成的吡唑束缚的苯并噻唑荧光团的发光性质,包括吸光度,激发,发射,摩尔消光系数,亮度和斯托克斯位移。


























 京公网安备 11010802027423号
京公网安备 11010802027423号