当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of 1-Aryl Benzo[5]helicenes Using BINOL-Derived Cationic Phosphonites as Ancillary Ligands.
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-09-08 , DOI: 10.1002/anie.202010021 Pablo Redero 1 , Thierry Hartung 1 , Jianwei Zhang 1 , Leo D M Nicholls 1 , Guo Zichen 1 , Martin Simon 1 , Christopher Golz 1 , Manuel Alcarazo 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-09-08 , DOI: 10.1002/anie.202010021 Pablo Redero 1 , Thierry Hartung 1 , Jianwei Zhang 1 , Leo D M Nicholls 1 , Guo Zichen 1 , Martin Simon 1 , Christopher Golz 1 , Manuel Alcarazo 1
Affiliation
|
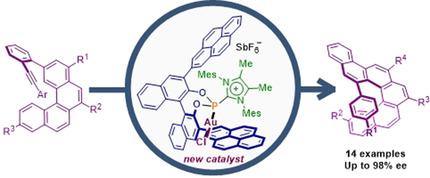
The synthesis of unprecedented BINOL‐derived cationic phosphonites is described. Through the use of these phosphanes as ancillary ligands in AuI catalysis, a highly regio‐ and enantioselective assembly of appropriately designed alkynes into 1‐(aryl)benzo[5]carbohelicenes is achieved. The modular synthesis of these ligands and the enhanced reactivity that they impart to AuI‐centers after coordination have been found to be the key features that allow an optimization of the reaction conditions until the desired benzo[5]helicenes are obtained with high yield and enantioselectivity.
中文翻译:

使用BINOL衍生的阳离子亚膦酸酯作为辅助配体,对映选择性合成1-芳基苯并[5]螺旋。
描述了前所未有的BINOL衍生的阳离子亚膦酸酯的合成。通过在Au I催化中使用这些膦作为辅助配体,可以将适当设计的炔烃高度区域和对映选择性组装成1-(芳基)苯并[5]碳杂环丁烯。这些配体的模块化合成以及配位后赋予Au I中心的增强反应性是关键特征,可优化反应条件,直到以高收率获得所需的苯并[5]螺旋烯为止对映选择性。
更新日期:2020-09-08
中文翻译:

使用BINOL衍生的阳离子亚膦酸酯作为辅助配体,对映选择性合成1-芳基苯并[5]螺旋。
描述了前所未有的BINOL衍生的阳离子亚膦酸酯的合成。通过在Au I催化中使用这些膦作为辅助配体,可以将适当设计的炔烃高度区域和对映选择性组装成1-(芳基)苯并[5]碳杂环丁烯。这些配体的模块化合成以及配位后赋予Au I中心的增强反应性是关键特征,可优化反应条件,直到以高收率获得所需的苯并[5]螺旋烯为止对映选择性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号