当前位置:
X-MOL 学术
›
J. Neuroimmunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A bibliometric evaluation of the top 100 cited natalizumab articles
Journal of Neuroimmunology ( IF 3.3 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.jneuroim.2020.577379 Francisco Javier García-Fernández , Alba Estela García-Fernández , Eduardo Nava , Julian Solis Garcia del Pozo , Ichiro Ikuta , Joaquin Jordan , Maria F. Galindo
Journal of Neuroimmunology ( IF 3.3 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.jneuroim.2020.577379 Francisco Javier García-Fernández , Alba Estela García-Fernández , Eduardo Nava , Julian Solis Garcia del Pozo , Ichiro Ikuta , Joaquin Jordan , Maria F. Galindo
|
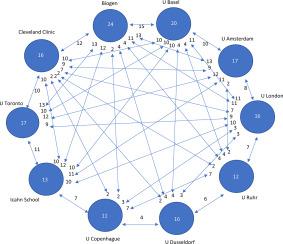
Natalizumab is being used in recurrent multiple sclerosis despite its history of market withdrawal due to lethal cases. We have carried out a bibliometric analysis of this drug from 1999 to February 2020 in order to assess the real impact of the use natalizumab with the goal to identify the key articles that sustain the current knowledge on the therapeutic possibilities of this compound. We have extracted from the Web of Science the top 100 most cited records (T100) and tabulated data on the journal, authors, publication year, number of citations, countries and institutions of publication, T100-records, citation density and citations per record of the works. The 100 most cited articles were selected from a total of 32,507 citations out of 2817 publications with an h-number of 74, 11.54 citations/publication, and a density of 1544.79 citations/year. Citations ranged from 63 of the paper placed in the 100th position (T100) to 1940 of the paper in the first position (T1). T2 was cited 888 times, and the difference in the number of citations between T1 and T2 was higher than that between T2 and T10. T1, T2 and T3 are clinical trials. When articles are arranged by institution and nationality having more than 10 T100 articles, biotechnology company Biogen and the USA, respectively, lead the ranking, but we also find that 8 out of 10 are academic European institutions. A co-authorship analysis reveals an intense collaborative activity between countries and institutions. We conclude that the clinical and academic communities have shown a sustained interest in natalizumab for the therapy of recurrent multiple sclerosis over the last 20 years.
中文翻译:

前 100 篇那他珠单抗文章的文献计量学评估
尽管 Natalizumab 曾因致命病例而退出市场,但它仍被用于复发性多发性硬化症。我们从 1999 年到 2020 年 2 月对该药物进行了文献计量分析,以评估使用那他珠单抗的实际影响,目的是确定支持当前有关该化合物治疗可能性的知识的关键文章。我们从 Web of Science 中提取了前 100 条被引用最多的记录 (T100),并列出了有关期刊、作者、出版年份、引用次数、出版国家和机构、T100 记录、引用密度和每条记录的引用数的数据。作品。引用次数最多的 100 篇文章是从 2817 篇出版物中的 32,507 篇引文中选出的,h 数为 74,引用次数为 11.54 次/发表,引用密度为 1544.79 次/年。引用范围从位于第 100 位 (T100) 的 63 篇论文到位于第 1 位 (T1) 的 1940 篇论文。T2 被引用 888 次,T1 和 T2 之间的被引次数差异高于 T2 和 T10。T1、T2和T3是临床试验。当文章按机构和国籍排列超过 10 篇 T100 文章时,生物技术公司 Biogen 和美国分别领先,但我们也发现 10 家中有 8 家是欧洲学术机构。共同作者分析揭示了国家和机构之间的激烈合作活动。我们得出的结论是,在过去 20 年中,临床和学术界对那他珠单抗治疗复发性多发性硬化症表现出持续的兴趣。
更新日期:2020-12-01
中文翻译:

前 100 篇那他珠单抗文章的文献计量学评估
尽管 Natalizumab 曾因致命病例而退出市场,但它仍被用于复发性多发性硬化症。我们从 1999 年到 2020 年 2 月对该药物进行了文献计量分析,以评估使用那他珠单抗的实际影响,目的是确定支持当前有关该化合物治疗可能性的知识的关键文章。我们从 Web of Science 中提取了前 100 条被引用最多的记录 (T100),并列出了有关期刊、作者、出版年份、引用次数、出版国家和机构、T100 记录、引用密度和每条记录的引用数的数据。作品。引用次数最多的 100 篇文章是从 2817 篇出版物中的 32,507 篇引文中选出的,h 数为 74,引用次数为 11.54 次/发表,引用密度为 1544.79 次/年。引用范围从位于第 100 位 (T100) 的 63 篇论文到位于第 1 位 (T1) 的 1940 篇论文。T2 被引用 888 次,T1 和 T2 之间的被引次数差异高于 T2 和 T10。T1、T2和T3是临床试验。当文章按机构和国籍排列超过 10 篇 T100 文章时,生物技术公司 Biogen 和美国分别领先,但我们也发现 10 家中有 8 家是欧洲学术机构。共同作者分析揭示了国家和机构之间的激烈合作活动。我们得出的结论是,在过去 20 年中,临床和学术界对那他珠单抗治疗复发性多发性硬化症表现出持续的兴趣。



























 京公网安备 11010802027423号
京公网安备 11010802027423号