Journal of Molecular Liquids ( IF 6 ) Pub Date : 2020-09-08 , DOI: 10.1016/j.molliq.2020.114243 Zhina Razaghi , Milad Rezaei
|
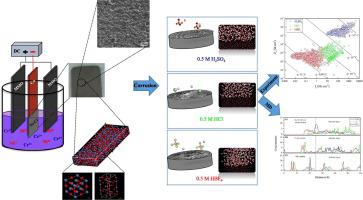
This study aimed to investigate the corrosion behavior of a relatively resistant nickel‑chromium coating in the presence of SO42−, Cl−, and BF4− ions. The electrochemical measurements including linear polarization, small amplitude cyclic voltammetry (SACV), and electrochemical noise (EN) analysis, were used, along with molecular dynamics simulation (MD) to evaluate the interactions between the coating and the corrosive ions. Results of the EN analysis showed that the charges of events exchanged in the HBF4 and HCl solutions were in the range of 1 μC to 1 mC; i.e., the coating was locally damaged in these media. However, the rates of pit progress in the coating exposed to BF4− and Cl− were not similar because the polarization resistances (Rp) of the coating in these two solutions were 1745.6 Ω·cm2 and 2766.2 Ω·cm2, respectively. The most negative adsorption energy, alongside the highest diffusion force of tetrafluoroborate ions, compared to chloride and sulfate, led to severe pitting corrosion of the coating. In the H2SO4 medium, the passivation mechanism prevailed over the pit growth, so that the Rp increased to 5747.3 Ω·cm2. Among the passive layers constructed on the coating surface, Cr2O3 (001) formed the low-energy chemical bonds with all three destructive species of SO42−, Cl−, and BF4−. Also, NiO (111) provided a compressed structure of Ni and Cr oxides, which could impede the penetration of corrosive ions in the alloyed coating.
中文翻译:

硫酸根,氯离子和四氟硼酸根离子与Ni-19 wt%Cr涂层相互作用的腐蚀机理:结合实验研究和分子动力学模拟
本研究的目的是调查中的存在的相对性的镍铬涂层的腐蚀行为SO 4 2-,氯-和BF 4 -的离子。电化学测量包括线性极化,小幅度循环伏安法(SACV)和电化学噪声(EN)分析,以及分子动力学模拟(MD)来评估涂层与腐蚀性离子之间的相互作用。EN分析结果表明,在HBF 4和HCl溶液中 交换的事件电荷在1μC到1 mC之间; 也就是说,涂层在这些介质中被局部损坏。然而,在涂层暴露于坑进展率BF 4 -和氯-不相似,因为极化电阻([R p在这两种解决方案的涂层的)为1745.6Ω·厘米2和2766.2Ω·厘米2,分别。与氯化物和硫酸盐相比,最负的吸附能以及四氟硼酸根离子的最高扩散力导致了涂层的严重点蚀。在H 2 SO 4介质中,钝化机制优先于坑的生长,因此R p增加到5747.3Ω· cm 2。之间的涂层表面上构成的无源层,铬2 ö 3形成的低能量化学键与所有三个物种破坏性(001)SO 4 2-,氯-和BF 4 - 。而且,NiO(111)提供了Ni和Cr氧化物的压缩结构,这可能会阻止腐蚀性离子在合金涂层中的渗透。


























 京公网安备 11010802027423号
京公网安备 11010802027423号