Journal of Allergy and Clinical Immunology ( IF 14.2 ) Pub Date : 2020-09-08 , DOI: 10.1016/j.jaci.2020.07.038 Yeon Duk Woo 1 , Jaemoon Koh 2 , Jae Sung Ko 1 , Sehui Kim 2 , Kyeong Cheon Jung 2 , Yoon Kyung Jeon 2 , Hye Young Kim 1 , Ho Lee 3 , Chang Woo Lee 4 , Doo Hyun Chung 5
|
Background
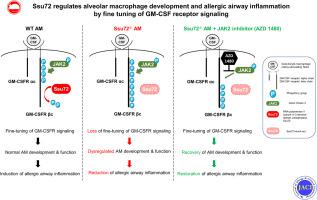
Fine-tuning of immune receptor signaling is critical for the development and functioning of immune cells. Moreover, GM-CSF receptor (GM-CSFR) signaling plays an essential role in the development of certain myeloid lineage cells, including alveolar macrophages (AMs). However, the significance of fine-tuning of GM-CSFR signaling in AMs and its relevance in allergic inflammation have not been reported.
Objective
Our aim was to explore whether phosphatase Ssu72, originally identified as a regulator of RNA polymerase II activity, regulates AM development and allergic airway inflammation by regulating GM-CSF signaling.
Methods
To address these issues, we generated LysM-CreSsu72fl/fl and Cd11c-CreSsu72fl/fl mice and used ovalbumin- or house dust mite–induced allergic asthma models.
Results
Following GM-CSF stimulation, Ssu72 directly bound to the GM-CSFR β-chain in AMs, preventing phosphorylation. Consistently, mature Ssu72-deficient AMs showed higher phosphorylation of the GM-CSFR β-chain and downstream molecules, which resulted in greater dysregulation of cell cycle, cell death, cell turnover, mitochondria-related metabolism, and LPS responsiveness in AMs than in mature wild-type AMs. The dysregulation was restored by using a Janus kinase 2 inhibitor, which reduced GM-CSFR β-chain phosphorylation. LysM-CreSsu72fl/fl mice exhibited deficits in development and maturation of AMs, which were also seen postnatally in Cd11c-CreSsu72fl/fl mice. Furthermore, LysM-CreSsu72fl/fl mice were less responsive to ovalbumin- or house dust mite–induced allergic asthma models than the control mice were; however, their responsiveness was restored by adoptive transfer of JAK2 inhibitor–pretreated mature Ssu72-deficient AMs.
Conclusion
Our results demonstrate that Ssu72 fine-tunes GM-CSFR signaling by both binding to and reducing phosphorylation of GM-CSFR β-chain, thereby regulating the development, maturation, and mitochondrial functions of AMs and allergic airway inflammation.
中文翻译:

Ssu72 通过微调 GM-CSF 受体信号调节肺泡巨噬细胞发育和过敏性气道炎症
背景
免疫受体信号的微调对于免疫细胞的发育和功能至关重要。此外,GM-CSF 受体 (GM-CSFR) 信号在某些髓系细胞的发育中起着重要作用,包括肺泡巨噬细胞 (AMs)。然而,尚未报道微调 AMs 中 GM-CSFR 信号的重要性及其与过敏性炎症的相关性。
客观的
我们的目的是探索磷酸酶 Ssu72(最初被确定为 RNA 聚合酶 II 活性的调节剂)是否通过调节 GM-CSF 信号传导来调节 AM 发展和过敏性气道炎症。
方法
为了解决这些问题,我们生成了 LysM-Cre Ssu 72 fl/fl和 Cd11c-Cre Ssu72 fl/fl小鼠,并使用了卵清蛋白或屋尘螨诱导的过敏性哮喘模型。
结果
在 GM-CSF 刺激后,Ssu72 直接与 AMs 中的 GM-CSFR β 链结合,防止磷酸化。一致地,成熟的 Ssu72 缺陷型 AMs 显示出更高的 GM-CSFR β 链和下游分子的磷酸化,这导致 AMs 中细胞周期、细胞死亡、细胞更新、线粒体相关代谢和 LPS 反应性的失调比成熟野生型 AM。通过使用 Janus 激酶 2 抑制剂恢复失调,该抑制剂降低了 GM-CSFR β-链磷酸化。LysM-Cre Ssu 72 fl/fl小鼠表现出 AMs 发育和成熟的缺陷,这在 Cd11c-Cre Ssu72 fl/fl小鼠出生后也可见。此外,LysM-Cre Ssu 72 fl/fl与对照小鼠相比,小鼠对卵清蛋白或屋尘螨诱发的过敏性哮喘模型的反应较弱;然而,通过 JAK2 抑制剂预处理的成熟 Ssu72 缺陷 AMs 的过继转移恢复了它们的反应性。
结论
我们的结果表明,Ssu72 通过结合和减少 GM-CSFR β 链的磷酸化来微调 GM-CSFR 信号,从而调节 AMs 和过敏性气道炎症的发育、成熟和线粒体功能。


























 京公网安备 11010802027423号
京公网安备 11010802027423号