Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease ( IF 6.2 ) Pub Date : 2020-09-08 , DOI: 10.1016/j.bbadis.2020.165959 Laura Solé 1 , Jacy L Wagnon 2 , Michael M Tamkun 3
|
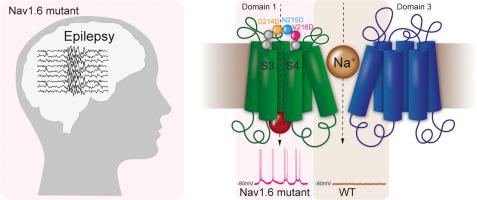
The voltage-gated sodium channel Nav1.6 is associated with more than 300 cases of epileptic encephalopathy. Nav1.6 epilepsy-causing mutations are spread over the entire channel's structure and only 10% of mutations have been characterized at the molecular level, with most of them being gain of function mutations. In this study, we analyzed three previously uncharacterized Nav1.6 epilepsy-causing mutations: G214D, N215D and V216D, located within a mutation hot-spot at the S3-S4 extracellular loop of Domain1. Voltage clamp experiments showed a 6–16 mV hyperpolarizing shift in the activation mid-point for all three mutants. V216D presented the largest shift along with decreased current amplitude, enhanced inactivation and a lack of persistent current. Recordings at hyperpolarized potentials indicated that all three mutants presented gating pore currents. Furthermore, trafficking experiments performed in cultured hippocampal neurons demonstrated that the mutants trafficked properly to the cell surface, with no significant differences regarding surface expression within the axon initial segment or soma compared to wild-type. These trafficking data suggest that the disease-causing consequences are due to only changes in the biophysical properties of the channel. Interestingly, the patient carrying the V216D mutation, which is the mutant with the greatest electrophysiological changes as compared to wild-type, exhibited the most severe phenotype. These results emphasize that these mutations will mandate unique treatment approaches, for normal sodium channel blockers may not work given that the studied mutations present gating pore currents. This study emphasizes the importance of molecular characterization of disease-causing mutations in order to improve the pharmacological treatment of patients.
中文翻译:

导致早期婴儿癫痫性脑病的三个 Nav1.6 突变的功能分析。
电压门控钠通道 Na v 1.6 与 300 多例癫痫性脑病有关。Na v 1.6 引起癫痫的突变遍布整个通道结构,只有 10% 的突变在分子水平上被表征,其中大部分是功能获得性突变。在这项研究中,我们分析了三个以前未表征的 Na v1.6 导致癫痫的突变:G214D、N215D 和 V216D,位于 Domain1 的 S3-S4 细胞外环的突变热点内。电压钳实验表明,所有三个突变体的激活中点都有 6-16 mV 的超极化转变。V216D 表现出最大的转变,伴随着电流幅度的降低、失活增强和持续电流的缺乏。超极化电位的记录表明所有三个突变体都存在门控孔电流。此外,在培养的海马神经元中进行的贩运实验表明,突变体正确地转运到细胞表面,与野生型相比,轴突初始段或体细胞内的表面表达没有显着差异。这些贩运数据表明,导致疾病的后果仅是由于通道的生物物理特性发生了变化。有趣的是,携带 V216D 突变(与野生型相比电生理变化最大的突变体)的患者表现出最严重的表型。这些结果强调,这些突变将要求采用独特的治疗方法,因为所研究的突变存在门控孔电流,因此正常的钠通道阻滞剂可能不起作用。本研究强调了对致病突变进行分子表征的重要性,以改善患者的药物治疗。这是与野生型相比具有最大电生理变化的突变体,表现出最严重的表型。这些结果强调,这些突变将要求采用独特的治疗方法,因为所研究的突变存在门控孔电流,因此正常的钠通道阻滞剂可能不起作用。本研究强调了对致病突变进行分子表征的重要性,以改善患者的药物治疗。这是与野生型相比具有最大电生理变化的突变体,表现出最严重的表型。这些结果强调,这些突变将要求采用独特的治疗方法,因为所研究的突变存在门控孔电流,因此正常的钠通道阻滞剂可能不起作用。本研究强调了对致病突变进行分子表征的重要性,以改善患者的药物治疗。



























 京公网安备 11010802027423号
京公网安备 11010802027423号