Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Interfacial thermodynamics of spherical nanodroplets: molecular understanding of surface tension via a hydrogen bond network.
Nanoscale ( IF 6.7 ) Pub Date : 2020-09-07 , DOI: 10.1039/d0nr04533k QHwan Kim 1 , Wonho Jhe 1
Nanoscale ( IF 6.7 ) Pub Date : 2020-09-07 , DOI: 10.1039/d0nr04533k QHwan Kim 1 , Wonho Jhe 1
Affiliation
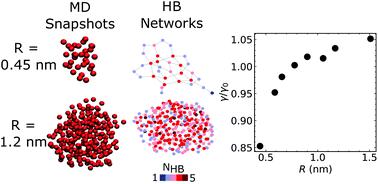
|
Surface tension plays a ubiquitous role in phase transitions including condensation or evaporation of atmospheric liquid droplets. In particular, understanding of interfacial thermodynamics of the critical nucleus of 1 nm scale is important for molecular characterization of the activation energy barrier of nucleation. Here, we investigate surface tension of spherical nanodroplets with both molecular dynamics and density functional theory and find that surface tension decreases appreciably below 1 nm radius, whose analytical expression is consistently derived from the classic Tolman's equation. In particular, the free energy analysis of nanodroplets shows that the change of surface tension originates dominantly from the configurational energy of interfacial molecules, which is evidenced by the increasingly disrupted hydrogen bond network as the droplet size decreases. Our result can be applied to the interface-related phenomena associated with molecular fluctuations such as biomolecule adsorption at the sub-nm scale where macroscopic thermodynamic quantities are ill-defined.
中文翻译:

球形纳米液滴的界面热力学:通过氢键网络对表面张力的分子理解。
表面张力在包括凝结或大气液滴蒸发的相变中起普遍作用。特别是,了解1 nm尺度的关键核的界面热力学对于成核活化能垒的分子表征非常重要。在这里,我们用分子动力学和密度泛函理论研究球形纳米液滴的表面张力,发现在1 nm半径以下表面张力明显降低,其解析表达式始终来自经典的Tolman方程。尤其是,对纳米液滴的自由能分析表明,表面张力的变化主要源自界面分子的结构能,随着液滴尺寸的减小,氢键网络的不断破坏证明了这一点。我们的结果可以应用于与分子波动相关的界面相关现象,例如亚纳米尺度上的生物分子吸附,其中宏观热力学量不确定。
更新日期:2020-09-24
中文翻译:

球形纳米液滴的界面热力学:通过氢键网络对表面张力的分子理解。
表面张力在包括凝结或大气液滴蒸发的相变中起普遍作用。特别是,了解1 nm尺度的关键核的界面热力学对于成核活化能垒的分子表征非常重要。在这里,我们用分子动力学和密度泛函理论研究球形纳米液滴的表面张力,发现在1 nm半径以下表面张力明显降低,其解析表达式始终来自经典的Tolman方程。尤其是,对纳米液滴的自由能分析表明,表面张力的变化主要源自界面分子的结构能,随着液滴尺寸的减小,氢键网络的不断破坏证明了这一点。我们的结果可以应用于与分子波动相关的界面相关现象,例如亚纳米尺度上的生物分子吸附,其中宏观热力学量不确定。


























 京公网安备 11010802027423号
京公网安备 11010802027423号