Medicinal Chemistry ( IF 2.3 ) Pub Date : 2020-08-31 , DOI: 10.2174/1573406415666190620144404 Mona A. Hosny 1 , Yasser H. Zaki 2 , Wafaa A. Mokbel 1 , Abdou O. Abdelhamid 3
|
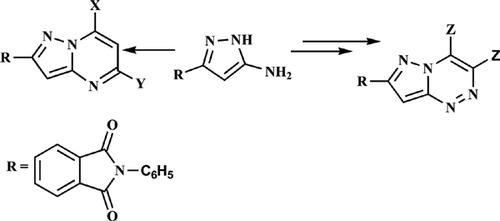
Background: Pyrazole and its derivatives are known to exhibit significant biological and pharmacological activities such as anticancer, anti-inflammatory, antioxidant, antibacterial, analgesic, antiviral, antimicrobial, antifungal, anti-glycemic, antiamoebic, and antidepressive. Considering the immense biological properties, pyrazole is one of the most widely studied nitrogen- containing heterocyclic nuclei. Fused pyrazole derivatives are composed of the pyrazole nucleus attached to other heterocyclic moieties.
Objective: The objective of this article is the synthesis of some new pyrazolo[1,5-a]pyrimidine and pyrazolo[5,1-c]1,2,4-triazine derivatives with potential anticancer and antimicrobial activities.
Methods: The in vitro growth inhibitory rates (%) and inhibitory growth activity (as measured by IC50) of the newly synthesized compounds were determined against the MCF-7 human breast carcinoma cell line in comparison with the well-known anticancer drug doxorubicin as the standard, using the MTT viability assay. The data generated were used to plot a dose-response curve from which the concentration (μM) of tested compounds required to kill 50% of the cell population (IC50) was determined. Cytotoxic activity was expressed as the mean IC50 of three independent experiments. The difference between inhibitory activities of all compounds with different concentrations was statistically significant p < 0.001. All compounds were structurally characterized by different spectroscopic techniques EI-MS, 1H-NMR, and 13C-NMR, and evaluated for their anticancer and antimicrobial activities (antibacterial and antifungal).
Results: Several pyrazolo[1,5-a]pyrimidine derivatives were synthesized from the reaction of 2-(4- (5-amino-1H-pyrazol-3-yl)phenyl)-1H-indene-1,3(2H)-dione with the appropriate active methylene compounds in boiling ethanol. Also, pyrazolo[5,1-c]triazines were obtained through the reaction of 2-(4-(5-(chlorodiazenyl)-1H-pyrazol-3-yl)phenyl)-1H-indene-1,3(2H)-dione with various active methylene compounds in ethanol containing sodium acetate at 0-5 °C. The structures of the newly synthesized compounds were elucidated on the basis of elemental analysis, spectral data, and alternative synthetic routes whenever possible. The newly synthesized compounds were evaluated for their antitumor activity against a breast cancer cell line (MCF-7) and a human colon cancer cell line (HCT-116). The results revealed that the tested compounds showed high variation in the inhibitory growth rates and activities against the tested tumor cell lines. All newly synthesized compounds screen towards microorganisms e.g. Gram-negative bacteria, Gram-positive bacteria, and Fungi.
Conclusion: 2-(4-(5-Amino-1H-pyrazol-3-yl)phenyl)isoindoline-1,3-dione proved to be a useful precursor for the synthesis of various pyrazolo[1,5-a]pyrimidine and pyrazolo[5,1-c]-1,2,4- triazines. The structures of the newly synthesized compounds were confirmed by spectral data and elemental analyses. The newly synthesized compounds were tested in vitro against the MCF-7, HCT-116 human cancer cell line and compared with doxorubicin as the standard, using the MTT viability assay. Most of the tested compounds were found to have moderate to high anticancer activity.
中文翻译:

某些新型吡唑并[1,5-a]嘧啶和吡唑并[5,1-c] 1,2,4-三嗪的合成,表征,抗菌活性和抗癌性
背景:吡唑及其衍生物具有显着的生物学和药理活性,例如抗癌,抗炎,抗氧化剂,抗菌,镇痛,抗病毒,抗微生物,抗真菌,抗血糖,抗阿米巴和抗抑郁作用。考虑到巨大的生物学特性,吡唑是研究最广泛的含氮杂环核之一。熔融的吡唑衍生物由与其他杂环部分连接的吡唑核组成。
目的:本文的目的是合成一些具有潜在抗癌和抗菌活性的吡唑并[1,5-a]嘧啶和吡唑并[5,1-c] 1,2,4-三嗪衍生物。
方法:与著名的抗癌药物阿霉素相比,测定新合成的化合物对MCF-7人乳腺癌细胞系的体外生长抑制率(%)和抑制生长活性(通过IC50测定)。标准,使用MTT生存力分析。产生的数据用于绘制剂量反应曲线,从中确定杀死50%细胞群体(IC50)所需的被测化合物的浓度(μM)。细胞毒性活性表示为三个独立实验的平均IC50。具有不同浓度的所有化合物的抑制活性之间的差异具有统计学显着性,p <0.001。所有化合物的结构均采用不同的光谱技术EI-MS,1H-NMR和13C-NMR表征,
结果:由2-(4-(5-氨基-1H-吡唑-3-基)苯基)-1H-茚-1,3(2H)反应合成了几种吡唑并[1,5-a]嘧啶衍生物-二酮与适当的活性亚甲基化合物在沸腾的乙醇中。另外,通过2-(4-(5-(氯二氮烯基)-1H-吡唑-3-基)苯基)-1H-茚-1,3(2H)反应获得吡唑并[5,1-c]三嗪。 -二酮与各种活性亚甲基化合物在0-5°C的含乙酸钠的乙醇中。根据元素分析,光谱数据和可能的替代合成路线,阐明了新合成化合物的结构。评价新合成的化合物对乳腺癌细胞系(MCF-7)和人结肠癌细胞系(HCT-116)的抗肿瘤活性。结果表明,所测试的化合物在抑制生长速率和针对所测试的肿瘤细胞系的活性方面显示出高的变化。所有新合成的化合物都针对微生物,例如革兰氏阴性细菌,革兰氏阳性细菌和真菌。
结论:2-(4-(5-氨基-1H-吡唑-3-基)苯基)异吲哚啉-1,3-二酮被证明是合成各种吡唑并[1,5-a]嘧啶和吡唑并[5,1-c] -1,2,4-三嗪。通过光谱数据和元素分析证实了新合成化合物的结构。使用MTT活力测定法,对新合成的化合物针对MCF-7,HCT-116人癌细胞系进行了体外测试,并与阿霉素为标准品进行了比较。发现大多数测试化合物具有中等至高的抗癌活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号