Medicinal Chemistry ( IF 2.3 ) Pub Date : 2020-08-31 , DOI: 10.2174/1573406415666190716153425 Huda K. Mahmoud 1 , Hanadi A. Katouah 2 , Marwa F. Harras 3 , Thoraya A. Farghaly 2
|
Background: One of the most successful reagents used in the synthesis of the reactive enaminone is DMF-DMA, but it is very expensive with harmful effects on the human health and reacts with special compounds to generate the enaminone such as active methylene centers.
Aim: In this article, we synthesized a new ketenaminal by simple method with inexpensive reagents (through desulfurization in diphenylether).
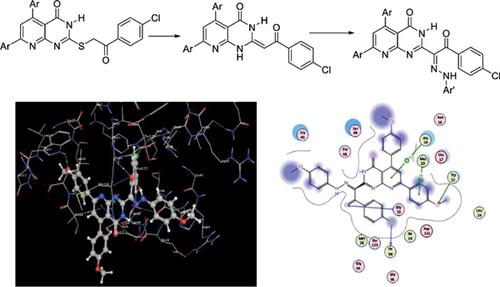
Methods: Thus, a novel reactive ketenaminal (enaminone) was synthesized from the desulfurization of 2-((2-(4-chlorophenyl)-2-oxoethyl)thio)-5,7-bis(4-methoxyphenyl)pyrido[2,3-d]pyrimidin- 4(3H)-one with diphenylether. The starting keteneaminal was coupled with diazotized anilines via the known coupling conditions to give a new series of 2-(4-chlorophenyl)-1-(2-(arylhydrazono)-2- oxoethyl)-5,7-bis(4-methoxy-phenyl)pyrido[2,3-d]pyrimidin-4(1H)-ones.
Results: The structures of the new compounds were elucidated based on their IR, 1H-NMR, 13CNMR, and Mass spectra. Moreover, the potency of these compounds as antimicrobial agents has been evaluated. The results showed that some of the products have high activity nearly equal to that of the used standard antibiotic. Additionally, the docking study was done to get the binding mode of the synthesized compounds with the binding site of the DHFR enzyme. The results of molecular docking of the synthesized arylhydrazono compounds are able to fit in DHFR binding site with binding energies ranging from -4.989 to -8.178 Kcal/mol.
Conclusion: Our goal was achieved in this context by the synthesis of new ketenaminal from inexpensive reagents, which was utilized in the preparation of bioactive arylhydrazone derivatives.
中文翻译:

一种新型的反应性酮戊胺:产品的合成,偶联反应,互变异构研究,对接和抗菌评估
背景:用于合成反应性烯胺酮的最成功的试剂之一是DMF-DMA,但它非常昂贵,会对人体健康造成有害影响,并且会与特殊化合物反应生成烯胺酮,例如活性亚甲基中心。
目的:在本文中,我们使用廉价的试剂(通过在二苯醚中进行脱硫)通过简单的方法合成了一种新的烯酮胺。
方法:因此,通过对2-((2-(4-氯苯基)-2-氧代乙基)硫基)-5,7-双(4-甲氧基苯基)吡啶基进行脱硫合成了一种新型反应性烯胺酮(烯胺),[2, 3-d]嘧啶-4(3H)-1与二苯醚。通过已知的偶合条件,将起始烯酮醛与重氮化苯胺偶合,得到一系列新的2-(4-氯苯基)-1-(2-(芳基肼基)-2-氧代乙基)-5,7-双(4-甲氧基) -苯基)吡啶并[2,3-d]嘧啶-4(1H)-一个。
结果:根据新化合物的IR,1H-NMR,13CNMR和质谱对结构进行了阐明。此外,已经评估了这些化合物作为抗微生物剂的效力。结果表明,某些产品具有与所用标准抗生素几乎相同的高活性。另外,进行对接研究以获得合成的化合物与DHFR酶的结合位点的结合模式。合成的芳基肼基化合物的分子对接的结果能够以-4.989至-8.178 Kcal / mol的结合能适合DHFR结合位点。
结论:在此背景下,我们的目标是通过从廉价的试剂合成新的酮亚胺,用于制备生物活性的芳基ena衍生物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号