当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Site-Selective Modification of Peptides and Proteins via Interception of Free Radical-Mediated Dechalcogenation.
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-09-06 , DOI: 10.1002/anie.202006260 Rhys C Griffiths 1 , Frances R Smith 1 , Jed E Long 1 , Huw E L Williams 2 , Robert Layfield 3 , Nicholas J Mitchell 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-09-06 , DOI: 10.1002/anie.202006260 Rhys C Griffiths 1 , Frances R Smith 1 , Jed E Long 1 , Huw E L Williams 2 , Robert Layfield 3 , Nicholas J Mitchell 1
Affiliation
|
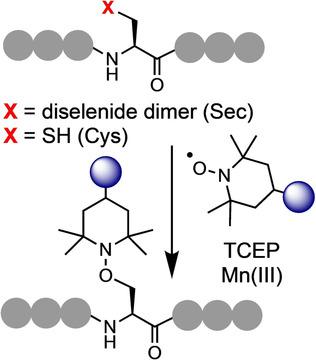
The development of site‐selective chemistry targeting the canonical amino acids enables the controlled installation of desired functionalities into native peptides and proteins. Such techniques facilitate the development of polypeptide conjugates to advance therapeutics, diagnostics, and fundamental science. We report a versatile and selective method to functionalize peptides and proteins through free‐radical‐mediated dechalcogenation. By exploiting phosphine‐induced homolysis of the C−Se and C−S bonds of selenocysteine and cysteine, respectively, we demonstrate the site‐selective installation of groups appended to a persistent radical trap. The reaction is rapid, operationally simple, and chemoselective. The resulting aminooxy linker is stable under a variety of conditions and selectively cleavable in the presence of a low‐oxidation‐state transition metal. We have explored the full scope of this reaction using complex peptide systems and a recombinantly expressed protein.
中文翻译:

通过拦截自由基介导的脱十氢作用对肽和蛋白质进行位点选择性修饰。
针对经典氨基酸的位点选择性化学技术的发展实现了将所需功能可控地安装到天然肽和蛋白质中。这样的技术促进了多肽缀合物的开发,以促进治疗,诊断和基础科学。我们报道了一种通过自由基介导的脱硫作用使肽和蛋白质功能化的通用且选择性的方法。通过分别利用磷化氢诱导的硒代半胱氨酸和半胱氨酸的C-Se和C-S键的均质化,我们证明了对持久性自由基陷阱附加的基团的位点选择性安装。该反应是快速的,操作简单的和化学选择性的。所得的氨氧基连接子在多种条件下均稳定,并且在低氧化态过渡金属存在下可选择性裂解。我们已经使用复杂的肽系统和重组表达的蛋白质探索了该反应的全部范围。
更新日期:2020-09-06
中文翻译:

通过拦截自由基介导的脱十氢作用对肽和蛋白质进行位点选择性修饰。
针对经典氨基酸的位点选择性化学技术的发展实现了将所需功能可控地安装到天然肽和蛋白质中。这样的技术促进了多肽缀合物的开发,以促进治疗,诊断和基础科学。我们报道了一种通过自由基介导的脱硫作用使肽和蛋白质功能化的通用且选择性的方法。通过分别利用磷化氢诱导的硒代半胱氨酸和半胱氨酸的C-Se和C-S键的均质化,我们证明了对持久性自由基陷阱附加的基团的位点选择性安装。该反应是快速的,操作简单的和化学选择性的。所得的氨氧基连接子在多种条件下均稳定,并且在低氧化态过渡金属存在下可选择性裂解。我们已经使用复杂的肽系统和重组表达的蛋白质探索了该反应的全部范围。



























 京公网安备 11010802027423号
京公网安备 11010802027423号