当前位置:
X-MOL 学术
›
Proteins Struct. Funct. Bioinform.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
MD simulation reveals differential binding of Cm(III) and Th(IV) with serum transferrin at acidic pH.
Proteins: Structure, Function, and Bioinformatics ( IF 2.9 ) Pub Date : 2020-09-06 , DOI: 10.1002/prot.26006 Lokpati Mishra 1, 2 , Mahesh Sundararajan 3 , Tusar Bandyopadhyay 2, 3
Proteins: Structure, Function, and Bioinformatics ( IF 2.9 ) Pub Date : 2020-09-06 , DOI: 10.1002/prot.26006 Lokpati Mishra 1, 2 , Mahesh Sundararajan 3 , Tusar Bandyopadhyay 2, 3
Affiliation
|
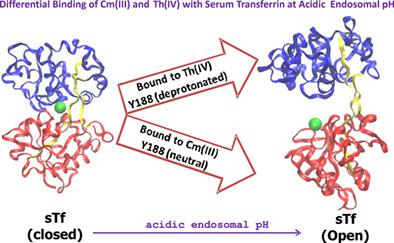
The iron carrier human serum transferrin (sTf) is known to transport other metals, including some actinides (An). Radiotoxic An are routinely involved in the nuclear fuel cycle and the possibility of their accidental exposure cannot be ruled out. Understanding An interaction with sTf assumes a greater significance for the development of safe and efficacious chelators for their removal from the blood stream. Here we report several 100 ns equilibrium MD simulations of Cm(III)‐ and Th(IV)‐loaded sTf at various protonation states of the protein to explore the possibility of the two An ions release and speciation. The results demonstrate variation in protonation state of dilysine pair (K206 and K296) and the tyrosine (Y188) residue is necessary for the opening of Cm(III)‐bound protein and the release of the ion. For the tetravalent thorium, protonation of dilysine pair suffices to cause conformational changes of protein. However, in none of the protonation states, Th(IV) releases from sTf because of its strong electrostatic interaction with D63 in the first shell of the sTf binding cleft. Analysis of hydrogen bond, water bridge, and the evaluation of potential of mean forces of the An ions' release from sTf, substantiate the differential behavior of Cm(III) and Th(IV) at endosomal pH. The results provide insight in the regulation of Cm(III) and Th(IV) bioavailability that may prove useful for effective design of their decorporating agents and as well may help the future design of radiotherapy based on tetravalent ions.
中文翻译:

MD模拟揭示了在酸性pH下Cm(III)和Th(IV)与血清转铁蛋白的差异结合。
已知铁载体人血清转铁蛋白(sTf)可以运输其他金属,包括某些act系元素(An)。放射性物质通常参与核燃料循环,因此不能排除其意外暴露的可能性。了解与sTf的相互作用对于开发安全有效的螯合剂以将其从血流中清除具有更大的意义。在这里,我们报告了Cm(III)和Th(IV)加载的sTf在蛋白质的各种质子化状态下的100 ns平衡MD模拟,以探索两种An离子释放和形成的可能性。结果表明,二赖氨酸对(K206和K296)的质子化状态发生变化,酪氨酸(Y188)残基对于Cm(III)结合蛋白的打开和离子的释放是必需的。对于四价or,二赖氨酸对的质子化足以引起蛋白质的构象变化。但是,在任何质子化状态下,Th(IV)均会从sTf中释放出来,因为它与sTf结合裂隙的第一壳中的D63具有强的静电相互作用。氢键,水桥的分析以及从sTf释放An离子的平均力的潜力的评估,证实了内体pH值下Cm(III)和Th(IV)的差异行为。结果为Cm(III)和Th(IV)生物利用度的调节提供了见识,这可能被证明对其去装饰剂的有效设计有用,并且也可能有助于将来基于四价离子的放射治疗设计。Th(IV)从sTf中释放出来,因为它与sTf结合裂隙的第一个壳中的D63有很强的静电相互作用。氢键,水桥的分析以及从sTf释放An离子的平均力的潜力的评估,证实了内体pH值下Cm(III)和Th(IV)的差异行为。该结果提供了对Cm(III)和Th(IV)生物利用度调节的见解,这些证明可能对有效设计其脱孔剂有用,也可能有助于将来基于四价离子的放射治疗设计。Th(IV)从sTf中释放出来,因为它与sTf结合裂隙的第一个壳中的D63有很强的静电相互作用。氢键,水桥的分析以及从sTf释放An离子的平均力的潜力的评估,证实了内体pH值下Cm(III)和Th(IV)的差异行为。该结果提供了对Cm(III)和Th(IV)生物利用度调节的见解,这些证明可能对有效设计其脱孔剂有用,也可能有助于将来基于四价离子的放射治疗设计。
更新日期:2020-09-06
中文翻译:

MD模拟揭示了在酸性pH下Cm(III)和Th(IV)与血清转铁蛋白的差异结合。
已知铁载体人血清转铁蛋白(sTf)可以运输其他金属,包括某些act系元素(An)。放射性物质通常参与核燃料循环,因此不能排除其意外暴露的可能性。了解与sTf的相互作用对于开发安全有效的螯合剂以将其从血流中清除具有更大的意义。在这里,我们报告了Cm(III)和Th(IV)加载的sTf在蛋白质的各种质子化状态下的100 ns平衡MD模拟,以探索两种An离子释放和形成的可能性。结果表明,二赖氨酸对(K206和K296)的质子化状态发生变化,酪氨酸(Y188)残基对于Cm(III)结合蛋白的打开和离子的释放是必需的。对于四价or,二赖氨酸对的质子化足以引起蛋白质的构象变化。但是,在任何质子化状态下,Th(IV)均会从sTf中释放出来,因为它与sTf结合裂隙的第一壳中的D63具有强的静电相互作用。氢键,水桥的分析以及从sTf释放An离子的平均力的潜力的评估,证实了内体pH值下Cm(III)和Th(IV)的差异行为。结果为Cm(III)和Th(IV)生物利用度的调节提供了见识,这可能被证明对其去装饰剂的有效设计有用,并且也可能有助于将来基于四价离子的放射治疗设计。Th(IV)从sTf中释放出来,因为它与sTf结合裂隙的第一个壳中的D63有很强的静电相互作用。氢键,水桥的分析以及从sTf释放An离子的平均力的潜力的评估,证实了内体pH值下Cm(III)和Th(IV)的差异行为。该结果提供了对Cm(III)和Th(IV)生物利用度调节的见解,这些证明可能对有效设计其脱孔剂有用,也可能有助于将来基于四价离子的放射治疗设计。Th(IV)从sTf中释放出来,因为它与sTf结合裂隙的第一个壳中的D63有很强的静电相互作用。氢键,水桥的分析以及从sTf释放An离子的平均力的潜力的评估,证实了内体pH值下Cm(III)和Th(IV)的差异行为。该结果提供了对Cm(III)和Th(IV)生物利用度调节的见解,这些证明可能对有效设计其脱孔剂有用,也可能有助于将来基于四价离子的放射治疗设计。


























 京公网安备 11010802027423号
京公网安备 11010802027423号