Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2020-09-06 , DOI: 10.1016/j.bmc.2020.115743 Jean F R Ribeiro 1 , Lorenzo Cianni 1 , Chan Li 2 , Thomas G Warwick 2 , Daniela de Vita 1 , Fabiana Rosini 1 , Fernanda Dos Reis Rocho 1 , Felipe C P Martins 1 , Peter W Kenny 1 , Jeronimo Lameira 3 , Andrei Leitão 1 , Jonas Emsley 2 , Carlos A Montanari 1
|
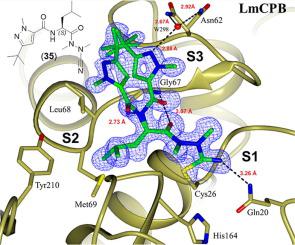
Leishmania mexicana is an obligate intracellular protozoan parasite that causes the cutaneous form of leishmaniasis affecting South America and Mexico. The cysteine protease LmCPB is essential for the virulence of the parasite and therefore, it is an appealing target for antiparasitic therapy. A library of nitrile-based cysteine protease inhibitors was screened against LmCPB to develop a treatment of cutaneous leishmaniasis. Several compounds are sufficiently high-affinity LmCPB inhibitors to serve both as starting points for drug discovery projects and as probes for target validation. A 1.4 Å X-ray crystal structure, the first to be reported for LmCPB, was determined for the complex of this enzyme covalently bound to an azadipeptide nitrile ligand. Mapping the structure-activity relationships for LmCPB inhibition revealed superadditive effects for two pairs of structural transformations. Therefore, this work advances our understanding of azadipeptidyl and dipeptidyl nitrile structure-activity relationships for LmCPB structure-based inhibitor design. We also tested the same series of inhibitors on related cysteine proteases cathepsin L and Trypanosoma cruzi cruzain. The modulation of these mammalian and protozoan proteases represents a new framework for targeting papain-like cysteine proteases.
中文翻译:

墨西哥利什曼原虫半胱氨酸蛋白酶B与高效氮杂二肽腈抑制剂复合物的晶体结构
墨西哥利什曼原虫是专一的细胞内原生动物寄生虫,引起皮肤利什曼病的形式,影响南美和墨西哥。半胱氨酸蛋白酶LmCPB对于寄生虫的毒力至关重要,因此,它是抗寄生虫治疗的诱人靶标。筛选针对LmCPB的基于腈的半胱氨酸蛋白酶抑制剂库,以开发治疗皮肤利什曼病的药物。几种化合物具有足够高的亲和力LmCPB抑制剂,既可以作为药物开发项目的起点,也可以作为目标验证的探针。确定了一种1.4ÅX射线晶体结构,这是LmCPB的首次报道,是该酶与氮杂二肽腈配体共价结合的复合物。映射LmCPB抑制的构效关系揭示了两对结构转化的超加和效应。因此,这项工作提高了我们对基于LmCPB结构的抑制剂设计的氮杂二肽基和二肽基腈结构-活性关系的理解。我们还对相关的半胱氨酸蛋白酶组织蛋白酶L和锥虫克鲁兹Cruzain。这些哺乳动物和原生动物蛋白酶的调节代表了针对木瓜蛋白酶样半胱氨酸蛋白酶的新框架。



























 京公网安备 11010802027423号
京公网安备 11010802027423号