当前位置:
X-MOL 学术
›
Environ. Sci.: Processes Impacts
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Products and mechanism of thermal decomposition of chlorpyrifos under inert and oxidative conditions.
Environmental Science: Processes & Impacts ( IF 5.5 ) Pub Date : 2020-09-05 , DOI: 10.1039/d0em00295j Nathan H Weber 1 , Sebastian P Stockenhuber , Emad Benhelal , Charles C Grimison , John A Lucas , John C Mackie , Michael Stockenhuber , Eric M Kennedy
Environmental Science: Processes & Impacts ( IF 5.5 ) Pub Date : 2020-09-05 , DOI: 10.1039/d0em00295j Nathan H Weber 1 , Sebastian P Stockenhuber , Emad Benhelal , Charles C Grimison , John A Lucas , John C Mackie , Michael Stockenhuber , Eric M Kennedy
Affiliation
|
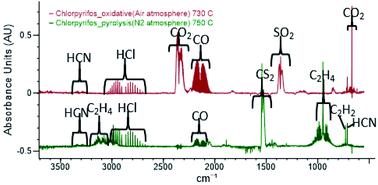
Chlorpyrifos (CPF) is a widely used pesticide; however, limited experimental work has been completed on its thermal decomposition. CPF is known to decompose into 3,5,6-trichloro-2-pyridinol (TCpyol) together with ethylene and HOPOS. Under oxidative conditions TCpyol can decompose into the dioxin-like 2,3,7,8-tetrachloro-[1,4]-dioxinodipyridine (TCDDPy). With CPF on the cusp of being banned in several jurisdictions worldwide, the question might arise as to how to safely eliminate large stockpiles of this pesticide. Thermal methods such as incineration or thermal desorption of pesticide-contaminated soils are often employed. To assess the safety of thermal methods, information about the toxicants arising from thermal treatment is essential. The present flow reactor study reports the products detected under inert and oxidative conditions from the decomposition of CPF representative of thermal treatments and of wildfires in CPF-contaminated vegetation. Ethylene and TCpyol are the initial products formed at temperatures between 550 and 650 °C, although the detection of HOPOS as a reaction product has proven to be elusive. During pyrolysis of CPF in an inert gas, the dominant sulfur-containing product detected from CPF is carbon disulfide. Quantum chemical analysis reveals that ethylene and HOPOS undergo a facile reaction to form thiirane (c-C2H4S) which subsequently undergoes ring opening reactions to form precursors of CS2. At elevated temperatures (>650 °C), TCpyol undergoes both decarbonylation and dehydroxylation reactions together with decomposition of its primary product, TCpyol. A substantial number of toxicants is observed, including HCN and several nitriles, including cyanogen. No CS2 is observed under oxidative conditions – sulfur dioxide is the fate of S in oxidation of CPF, and quantum chemical studies show that SO2 formation is initiated by the reaction between HOPOS and O2. The range of toxicants produced in thermal decomposition of CPF is summarised.
中文翻译:

毒死rif在惰性和氧化条件下的热分解产物和机理。
毒死rif(CPF)是一种广泛使用的农药。然而,关于其热分解的有限的实验工作已经完成。已知CPF与乙烯和HOPOS一起分解为3,5,6-三氯-2-吡啶醇(TCpyol)。在氧化条件下,TCpyol可分解为二恶英样的2,3,7,8-四氯-[1,4]-二恶二吡啶(TCDDPy)。随着CPF在全球多个司法管辖区被禁止的风口浪尖,可能会出现一个问题,即如何安全地消除这种农药的大量库存。经常采用热方法,如焚化或热解吸农药污染的土壤。为了评估热处理方法的安全性,有关热处理产生的有毒物质的信息至关重要。当前的流动反应堆研究报告了在惰性和氧化条件下从代表CPF污染的植被中的热处理和野火的CPF分解中检测到的产物。乙烯和TCpyol是在550至650°C的温度下形成的初始产物,尽管已证明难以检测到HOPOS作为反应产物。在惰性气体中CPF的热解过程中,从CPF中检测到的主要含硫产物是二硫化碳。量子化学分析表明,乙烯和HOPOS容易发生反应,形成硫杂环丁烷(cC 尽管已证明将HOPOS检测为反应产物是难以捉摸的。在惰性气体中CPF的热解过程中,从CPF中检测到的主要含硫产物是二硫化碳。量子化学分析表明,乙烯和HOPOS容易发生反应,形成硫杂环丁烷(cC 尽管已证明将HOPOS检测为反应产物是难以捉摸的。在惰性气体中CPF的热解过程中,从CPF中检测到的主要含硫产物是二硫化碳。量子化学分析表明,乙烯和HOPOS容易发生反应,形成硫杂环丁烷(cC2 H 4 S),其随后进行开环反应以形成CS 2的前体。在高温(> 650°C)下,TCpyol会同时经历脱羰和脱羟基反应,并分解其主要产物TCpyol。观察到大量的有毒物质,包括HCN和几种腈,包括氰。在氧化条件下未观察到CS 2-二氧化硫是CPF氧化中S的命运,量子化学研究表明SO 2的形成是由HOPOS与O 2之间的反应引发的。总结了CPF热分解过程中产生的毒物范围。
更新日期:2020-09-10
中文翻译:

毒死rif在惰性和氧化条件下的热分解产物和机理。
毒死rif(CPF)是一种广泛使用的农药。然而,关于其热分解的有限的实验工作已经完成。已知CPF与乙烯和HOPOS一起分解为3,5,6-三氯-2-吡啶醇(TCpyol)。在氧化条件下,TCpyol可分解为二恶英样的2,3,7,8-四氯-[1,4]-二恶二吡啶(TCDDPy)。随着CPF在全球多个司法管辖区被禁止的风口浪尖,可能会出现一个问题,即如何安全地消除这种农药的大量库存。经常采用热方法,如焚化或热解吸农药污染的土壤。为了评估热处理方法的安全性,有关热处理产生的有毒物质的信息至关重要。当前的流动反应堆研究报告了在惰性和氧化条件下从代表CPF污染的植被中的热处理和野火的CPF分解中检测到的产物。乙烯和TCpyol是在550至650°C的温度下形成的初始产物,尽管已证明难以检测到HOPOS作为反应产物。在惰性气体中CPF的热解过程中,从CPF中检测到的主要含硫产物是二硫化碳。量子化学分析表明,乙烯和HOPOS容易发生反应,形成硫杂环丁烷(cC 尽管已证明将HOPOS检测为反应产物是难以捉摸的。在惰性气体中CPF的热解过程中,从CPF中检测到的主要含硫产物是二硫化碳。量子化学分析表明,乙烯和HOPOS容易发生反应,形成硫杂环丁烷(cC 尽管已证明将HOPOS检测为反应产物是难以捉摸的。在惰性气体中CPF的热解过程中,从CPF中检测到的主要含硫产物是二硫化碳。量子化学分析表明,乙烯和HOPOS容易发生反应,形成硫杂环丁烷(cC2 H 4 S),其随后进行开环反应以形成CS 2的前体。在高温(> 650°C)下,TCpyol会同时经历脱羰和脱羟基反应,并分解其主要产物TCpyol。观察到大量的有毒物质,包括HCN和几种腈,包括氰。在氧化条件下未观察到CS 2-二氧化硫是CPF氧化中S的命运,量子化学研究表明SO 2的形成是由HOPOS与O 2之间的反应引发的。总结了CPF热分解过程中产生的毒物范围。


























 京公网安备 11010802027423号
京公网安备 11010802027423号