当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Difunctionalization of C–C σ Bonds Enabled by the Reaction of Bicyclo[1.1.0]butyl Boronate Complexes with Electrophiles: Reaction Development, Scope, and Stereochemical Origins
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-09-04 , DOI: 10.1021/jacs.0c07357 Steven H Bennett 1 , Alexander Fawcett 1 , Elliott H Denton 1 , Tobias Biberger 1 , Valerio Fasano 1 , Nils Winter 1 , Varinder K Aggarwal 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-09-04 , DOI: 10.1021/jacs.0c07357 Steven H Bennett 1 , Alexander Fawcett 1 , Elliott H Denton 1 , Tobias Biberger 1 , Valerio Fasano 1 , Nils Winter 1 , Varinder K Aggarwal 1
Affiliation
|
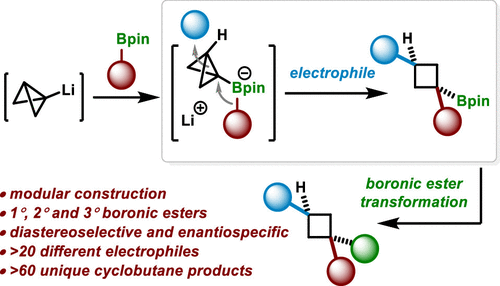
Difunctionalization reactions of C-C σ bonds have the potential to streamline access to molecules that would otherwise be difficult to prepare. However, the development of such reactions is challenging because C-C σ bonds are typically unreactive. Exploiting the high ring-strain energy of polycyclic carbocycles is a common strategy to weaken and facilitate the reaction of C-C σ bonds, but there are limited examples of highly strained C-C σ bonds being used in difunctionalization reactions. We demonstrate that highly strained bicyclo[1.1.0]butyl boronate complexes (strain energy: ca. 65 kcal/mol), which were prepared by reacting boronic esters with bicyclo[1.1.0]butyl lithium, react with electrophiles to achieve the diastereoselective difunctionalization of the strained central CC -bond of the bicyclo[1.1.0]butyl unit. The reaction shows broad substrate scope, with a range of different electrophiles and boronic esters being successfully employed to form a diverse set of 1,1,3-trisubstituted cyclobutanes (>50 examples) with high diastereoselectivity. The high diastereoselectivity observed has been rationalized based on a combination of experimental data and DFT calculations, which suggests that separate concerted and stepwise reaction mechanisms are operating depending upon the migrating substituent and electrophile used.
中文翻译:

双环[1.1.0]丁基硼酸酯配合物与亲电试剂反应使C-Cσ键双官能化:反应发展、范围和立体化学起源
CC σ 键的双官能化反应有可能简化对难以制备的分子的访问。然而,这种反应的发展具有挑战性,因为 CC σ 键通常是不活泼的。利用多环碳环的高环应变能是削弱和促进 CC σ 键反应的常用策略,但在双官能化反应中使用高应变 CC σ 键的例子有限。我们证明了通过硼酸酯与双环 [1.1.0] 丁基锂反应制备的高度应变的双环 [1.1.0] 丁基硼酸酯配合物(应变能:约 65 kcal/mol)与亲电子试剂反应以实现非对映选择性双环[1.1.0]丁基单元的应变中心C-C-键的双官能化。该反应显示出广泛的底物范围,一系列不同的亲电子试剂和硼酸酯被成功地用于形成具有高非对映选择性的多种 1,1,3-三取代环丁烷(>50 个实例)。基于实验数据和 DFT 计算的组合,观察到的高非对映选择性已被合理化,这表明单独的协调和逐步反应机制正在运行,具体取决于所使用的迁移取代基和亲电子试剂。
更新日期:2020-09-04
中文翻译:

双环[1.1.0]丁基硼酸酯配合物与亲电试剂反应使C-Cσ键双官能化:反应发展、范围和立体化学起源
CC σ 键的双官能化反应有可能简化对难以制备的分子的访问。然而,这种反应的发展具有挑战性,因为 CC σ 键通常是不活泼的。利用多环碳环的高环应变能是削弱和促进 CC σ 键反应的常用策略,但在双官能化反应中使用高应变 CC σ 键的例子有限。我们证明了通过硼酸酯与双环 [1.1.0] 丁基锂反应制备的高度应变的双环 [1.1.0] 丁基硼酸酯配合物(应变能:约 65 kcal/mol)与亲电子试剂反应以实现非对映选择性双环[1.1.0]丁基单元的应变中心C-C-键的双官能化。该反应显示出广泛的底物范围,一系列不同的亲电子试剂和硼酸酯被成功地用于形成具有高非对映选择性的多种 1,1,3-三取代环丁烷(>50 个实例)。基于实验数据和 DFT 计算的组合,观察到的高非对映选择性已被合理化,这表明单独的协调和逐步反应机制正在运行,具体取决于所使用的迁移取代基和亲电子试剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号