当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effects of Methyl Branching on the Properties and Performance of Furandioate-Adipate Copolyesters of Bio-Based Secondary Diols
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2020-09-04 , DOI: 10.1021/acssuschemeng.0c04513 Alastair Little 1 , Alessandro Pellis 1, 2 , James W. Comerford 1 , Edwin Naranjo-Valles 3 , Nema Hafezi 3 , Mark Mascal 3 , Thomas J. Farmer 1
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2020-09-04 , DOI: 10.1021/acssuschemeng.0c04513 Alastair Little 1 , Alessandro Pellis 1, 2 , James W. Comerford 1 , Edwin Naranjo-Valles 3 , Nema Hafezi 3 , Mark Mascal 3 , Thomas J. Farmer 1
Affiliation
|
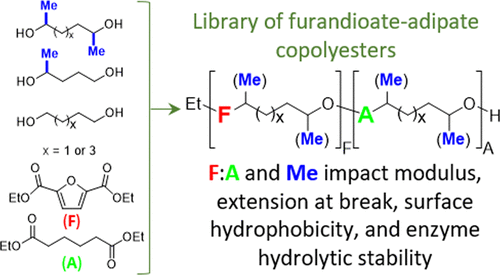
Furandioate-adipate copolyesters are an emerging class of bio-based biodegradable polymers with great potential to replace fossil-derived terephthalic acid-based copolyesters such as poly(butylene adipate-co-terephthalate) (PBAT). Furandioate-adipate polyesters have almost exclusively been prepared with conventional primary (1°) alcohol diols, while secondary (2°) alcohol diol monomers have largely been overlooked until now, despite preliminary observations that using methyl-branched diols increases the Tg of the resultant polyesters. Little is known of what impact the use of 2° alcohol diols has on other properties such as material strength, hydrophobicity, and rate of enzymatic hydrolysis—all key parameters for performance and end-of-life. To ascertain the effects of using 2° diols on the properties of furandioate-adipate copolyesters, a series of polymers from diethyl adipate (DEA) and 2,5-furandicarboxylic acid diethyl ester (FDEE) using different 1° and 2° alcohol diols was prepared. Longer transesterification times and greater excesses of diol (diol/diester molar ratio of 2:1) were found to be necessary to achieve Mws > 20 kDa using 2° alcohol diols. All copolyesters from 2° diols were entirely amorphous and exhibited higher Tgs than their linear equivalents from 1° diols. Compared to linear poly(1,4-butyleneadipate-co-1,4-butylenefurandioate), methyl-branched, poly(2,5-hexamethyleneadipate-co-2,5-hexamethylenefurandioate) (0:7:0.3 furandioate/adipate ratio) displayed both higher modulus (67.8 vs 19.1 MPa) and higher extension at break (89.7 vs 44.5 mm). All other methyl-branched copolyesters displayed lower modulus but retained higher extension at break compared with their linear analogues. Enzymatic hydrolysis studies using Humicola insolens cutinase revealed that copolyesters from 2° alcohol diols have significantly decreased rates of biodegradation than their linear equivalents synthesized using 1° alcohol diols, allowing for fine-tuning of polymer stability. Hydrophobicity, as revealed by water contact angles, was also found to generally increase through the introduction of methyl branching, demonstrating potential for these materials in coatings applications.
中文翻译:

甲基支化对基于生物的仲二醇呋喃二酸酯-己二酸酯共聚酯性能和性能的影响
Furandioate -己二酸的共聚酯是一类新兴的生物基可生物降解聚合物的具有巨大潜力替代化石来源的对苯二甲酸酸基共聚酯,例如聚(亚丁基己二酸酯共-terephthalate)(PBAT)。呋喃二酸酯-己二酸酯聚酯几乎完全由常规的伯(1°)醇二醇制备,而仲(2°)醇二醇单体至今仍被广泛忽略,尽管初步观察到使用甲基支化的二醇会增加T g。所得的聚酯。几乎没有人知道使用2°醇二醇对其他性能(例如材料强度,疏水性和酶水解速率)有什么影响,这些都是性能和寿命终止的所有关键参数。为了确定使用2°二醇对呋喃二酸酯-己二酸酯共聚酯性能的影响,使用了不同的1°和2°醇二醇制备了一系列己二酸二乙酯(DEA)和2,5-呋喃二甲酸二乙酯(FDEE)的聚合物。准备好了。发现更长的酯交换时间和更多过量的二醇(二醇/二酯摩尔比为2:1)对于使用2°醇二醇实现M w s> 20 kDa是必要的。来自2°二醇的所有共聚酯都是完全无定形的,并具有较高的T g比1°二醇的线性当量要大。相比线性聚(1,4- butyleneadipate-共-1,4- butylenefurandioate),甲基支链的,聚(2,5-亚hexamethyleneadipate-共-2,5- hexamethylenefurandioate)(0:7:0.3 furandioate /己二酸酯比率)显示出更高的模量(67.8对19.1 MPa)和更高的断裂伸长率(89.7对44.5 mm)。与它们的线性类似物相比,所有其他甲基支化共聚酯均显示出较低的模量,但保留较高的断裂伸长率。使用腐质霉的酶促水解研究角质酶显示,与使用1°醇二醇合成的线性等效物相比,来自2°醇二醇的共聚酯的生物降解速率显着降低,从而可以对聚合物的稳定性进行微调。还发现,通过水接触角显示的疏水性通常会由于引入甲基支化而增加,这证明了这些材料在涂料应用中的潜力。
更新日期:2020-09-28
中文翻译:

甲基支化对基于生物的仲二醇呋喃二酸酯-己二酸酯共聚酯性能和性能的影响
Furandioate -己二酸的共聚酯是一类新兴的生物基可生物降解聚合物的具有巨大潜力替代化石来源的对苯二甲酸酸基共聚酯,例如聚(亚丁基己二酸酯共-terephthalate)(PBAT)。呋喃二酸酯-己二酸酯聚酯几乎完全由常规的伯(1°)醇二醇制备,而仲(2°)醇二醇单体至今仍被广泛忽略,尽管初步观察到使用甲基支化的二醇会增加T g。所得的聚酯。几乎没有人知道使用2°醇二醇对其他性能(例如材料强度,疏水性和酶水解速率)有什么影响,这些都是性能和寿命终止的所有关键参数。为了确定使用2°二醇对呋喃二酸酯-己二酸酯共聚酯性能的影响,使用了不同的1°和2°醇二醇制备了一系列己二酸二乙酯(DEA)和2,5-呋喃二甲酸二乙酯(FDEE)的聚合物。准备好了。发现更长的酯交换时间和更多过量的二醇(二醇/二酯摩尔比为2:1)对于使用2°醇二醇实现M w s> 20 kDa是必要的。来自2°二醇的所有共聚酯都是完全无定形的,并具有较高的T g比1°二醇的线性当量要大。相比线性聚(1,4- butyleneadipate-共-1,4- butylenefurandioate),甲基支链的,聚(2,5-亚hexamethyleneadipate-共-2,5- hexamethylenefurandioate)(0:7:0.3 furandioate /己二酸酯比率)显示出更高的模量(67.8对19.1 MPa)和更高的断裂伸长率(89.7对44.5 mm)。与它们的线性类似物相比,所有其他甲基支化共聚酯均显示出较低的模量,但保留较高的断裂伸长率。使用腐质霉的酶促水解研究角质酶显示,与使用1°醇二醇合成的线性等效物相比,来自2°醇二醇的共聚酯的生物降解速率显着降低,从而可以对聚合物的稳定性进行微调。还发现,通过水接触角显示的疏水性通常会由于引入甲基支化而增加,这证明了这些材料在涂料应用中的潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号