当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis, Characterization, and Enthalpies of Formation of Uranium Substituted Zirconolites
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2020-09-04 , DOI: 10.1021/acsearthspacechem.0c00182 Tamilarasan Subramani 1 , Jason Baker 2 , Hongwu Xu 2 , Alexandra Navrotsky 1
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2020-09-04 , DOI: 10.1021/acsearthspacechem.0c00182 Tamilarasan Subramani 1 , Jason Baker 2 , Hongwu Xu 2 , Alexandra Navrotsky 1
Affiliation
|
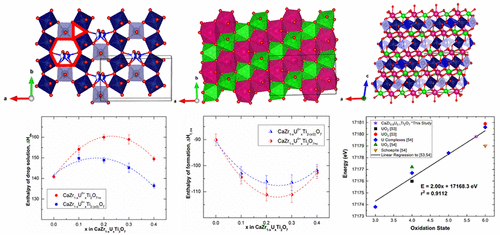
Uranium substituted zirconolite samples based on CaZrTi2O7 have been synthesized by a conventional solid-state method in air and structurally characterized by powder X-ray diffraction. The uranium substituted compounds crystallize in a monoclinic C2/m structure like the parent phase. The oxidation state of uranium has been confirmed to be mostly UVI by X-ray absorption spectroscopy (XAS). The enthalpies of formation from oxides (ΔH°f,ox) determined by high temperature oxide melt solution calorimetry of uranium substituted compounds are more exothermic than that of the parent CaZrTi2O7. The enthalpies of decomposition of uranium substituted zirconolites into perovskite (CaTiO3), UO3, and other binary oxides are endothermic by about 20 kJ/mol, implying that decomposition is probably thermodynamically unfavorable. The results show that zirconolite is a thermodynamically stable host for uranium in high level nuclear waste.
中文翻译:

铀取代锆钛矿的合成,表征和形成焓
基于CaZrTi 2 O 7的铀取代锆沸石样品已通过常规固态方法在空气中合成,并通过粉末X射线衍射对其结构进行了表征。铀取代的化合物像母相一样以单斜C 2 / m结构结晶。通过X射线吸收光谱法(XAS)已确认铀的氧化态大部分为U VI。用高温氧化物熔融溶液量热法测定的铀取代化合物的氧化物生成焓(ΔH ° f,ox)比母体CaZrTi 2 O 7放热大。。铀取代的锆钛矿分解成钙钛矿(CaTiO 3),UO 3和其他二元氧化物的分解焓是大约20 kJ / mol的吸热峰,这意味着分解可能在热力学上是不利的。结果表明,锆石是高含量核废料中铀的热力学稳定主体。
更新日期:2020-10-16
中文翻译:

铀取代锆钛矿的合成,表征和形成焓
基于CaZrTi 2 O 7的铀取代锆沸石样品已通过常规固态方法在空气中合成,并通过粉末X射线衍射对其结构进行了表征。铀取代的化合物像母相一样以单斜C 2 / m结构结晶。通过X射线吸收光谱法(XAS)已确认铀的氧化态大部分为U VI。用高温氧化物熔融溶液量热法测定的铀取代化合物的氧化物生成焓(ΔH ° f,ox)比母体CaZrTi 2 O 7放热大。。铀取代的锆钛矿分解成钙钛矿(CaTiO 3),UO 3和其他二元氧化物的分解焓是大约20 kJ / mol的吸热峰,这意味着分解可能在热力学上是不利的。结果表明,锆石是高含量核废料中铀的热力学稳定主体。


























 京公网安备 11010802027423号
京公网安备 11010802027423号