当前位置:
X-MOL 学术
›
J. Neurochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Oncostatin M induces hyperalgesic priming and amplifies signaling of cAMP to ERK by RapGEF2 and PKA
Journal of Neurochemistry ( IF 4.7 ) Pub Date : 2020-09-03 , DOI: 10.1111/jnc.15172 Anibal Garza Carbajal 1 , Andrea Ebersberger 2 , Alina Thiel 1 , Luiz Ferrari 3 , Jeremy Acuna 1 , Stephanie Brosig 1 , Joerg Isensee 1 , Katharina Moeller 1 , Maike Siobal 1 , Stefan Rose-John 4 , Jon Levine 3 , Hans-Georg Schaible 2 , Tim Hucho 1
Journal of Neurochemistry ( IF 4.7 ) Pub Date : 2020-09-03 , DOI: 10.1111/jnc.15172 Anibal Garza Carbajal 1 , Andrea Ebersberger 2 , Alina Thiel 1 , Luiz Ferrari 3 , Jeremy Acuna 1 , Stephanie Brosig 1 , Joerg Isensee 1 , Katharina Moeller 1 , Maike Siobal 1 , Stefan Rose-John 4 , Jon Levine 3 , Hans-Georg Schaible 2 , Tim Hucho 1
Affiliation
|
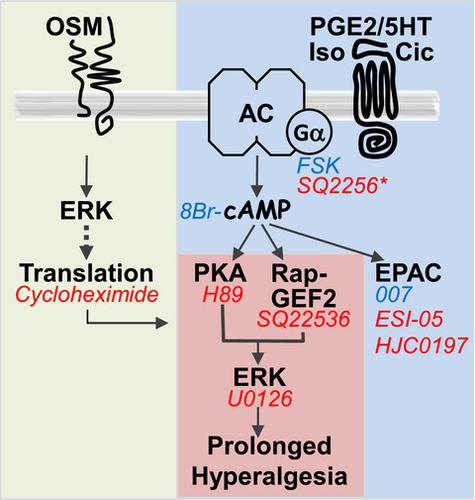
Hyperalgesic priming is characterized by enhanced nociceptor sensitization by pronociceptive mediators, prototypically PGE2. Priming has gained interest as a mechanism underlying the transition to chronic pain. Which stimuli induce priming and what cellular mechanisms are employed remains incompletely understood. In adult male rats, we present the cytokine Oncostatin M (OSM), a member of the IL-6 family, as an inducer of priming by a novel mechanism. We used a high content microscopy based approach to quantify the activation of endogenous PKA-II and ERK of thousands sensory neurons in culture. Incubation with OSM increased and prolonged ERK activation by agents that increase cAMP production such as PGE2, forskolin, and cAMP analogs. These changes were specific to IB4/CaMKIIα positive neurons, required protein translation, and increased cAMP-to-ERK signaling. In both, control and OSM-treated neurons, cAMP/ERK signaling involved RapGEF2 and PKA but not Epac. Similar enhancement of cAMP-to-ERK signaling could be induced by GDNF, which acts mostly on IB4/CaMKIIα-positive neurons, but not by NGF, which acts mostly on IB4/CaMKIIα-negative neurons. In vitro, OSM pretreatment rendered baseline TTX-R currents ERK-dependent and switched forskolin-increased currents from partial to full ERK-dependence in small/medium sized neurons. In summary, priming induced by OSM uses a novel mechanism to enhance and prolong coupling of cAMP/PKA to ERK1/2 signaling without changing the overall pathway structure.
中文翻译:

制瘤素 M 通过 RapGEF2 和 PKA 诱导痛觉过敏启动并放大 cAMP 到 ERK 的信号
痛觉过敏引发的特点是增强的伤害感受器敏化由伤害感受介质,原型 PGE 2。启动作为向慢性疼痛过渡的一种机制引起了人们的兴趣。哪些刺激诱导启动以及采用哪些细胞机制仍不完全清楚。在成年雄性大鼠中,我们将细胞因子制瘤素M (OSM)(IL-6 家族的成员)作为一种新机制引发的诱导剂。我们使用基于高内涵显微镜的方法来量化培养中数千个感觉神经元的内源性 PKA-II 和 ERK 的激活。与 OSM 一起孵育可通过增加 cAMP 产生的试剂(如 PGE 2)增加和延长 ERK 激活、毛喉素和 cAMP 类似物。这些变化特定于 IB4/CaMKIIα 阳性神经元,需要蛋白质翻译,并增加了 cAMP 到 ERK 的信号传导。在对照和 OSM 处理的神经元中,cAMP/ERK 信号传导涉及 RapGEF2 和 PKA,但不涉及 Epac。GDNF 可以诱导 cAMP 到 ERK 信号的类似增强,GDNF 主要作用于 IB4/CaMKIIα 阳性神经元,而不是 NGF,NGF 主要作用于 IB4/CaMKIIα 阴性神经元。在体外,OSM 预处理使基线 TTX-R 电流呈现 ERK 依赖性,并将毛喉素增加的电流从小/中型神经元中的部分 ERK 依赖性转变为完全依赖性。总之,OSM 诱导的启动使用一种新机制来增强和延长 cAMP/PKA 与 ERK1/2 信号传导的耦合,而不改变整体通路结构。
更新日期:2020-09-03
中文翻译:

制瘤素 M 通过 RapGEF2 和 PKA 诱导痛觉过敏启动并放大 cAMP 到 ERK 的信号
痛觉过敏引发的特点是增强的伤害感受器敏化由伤害感受介质,原型 PGE 2。启动作为向慢性疼痛过渡的一种机制引起了人们的兴趣。哪些刺激诱导启动以及采用哪些细胞机制仍不完全清楚。在成年雄性大鼠中,我们将细胞因子制瘤素M (OSM)(IL-6 家族的成员)作为一种新机制引发的诱导剂。我们使用基于高内涵显微镜的方法来量化培养中数千个感觉神经元的内源性 PKA-II 和 ERK 的激活。与 OSM 一起孵育可通过增加 cAMP 产生的试剂(如 PGE 2)增加和延长 ERK 激活、毛喉素和 cAMP 类似物。这些变化特定于 IB4/CaMKIIα 阳性神经元,需要蛋白质翻译,并增加了 cAMP 到 ERK 的信号传导。在对照和 OSM 处理的神经元中,cAMP/ERK 信号传导涉及 RapGEF2 和 PKA,但不涉及 Epac。GDNF 可以诱导 cAMP 到 ERK 信号的类似增强,GDNF 主要作用于 IB4/CaMKIIα 阳性神经元,而不是 NGF,NGF 主要作用于 IB4/CaMKIIα 阴性神经元。在体外,OSM 预处理使基线 TTX-R 电流呈现 ERK 依赖性,并将毛喉素增加的电流从小/中型神经元中的部分 ERK 依赖性转变为完全依赖性。总之,OSM 诱导的启动使用一种新机制来增强和延长 cAMP/PKA 与 ERK1/2 信号传导的耦合,而不改变整体通路结构。


























 京公网安备 11010802027423号
京公网安备 11010802027423号