Cellular Signalling ( IF 4.8 ) Pub Date : 2020-09-04 , DOI: 10.1016/j.cellsig.2020.109768 Ji Li 1 , Wei Ye 1 , Wenqin Xu 2 , Tianfang Chang 1 , Luning Zhang 1 , Jiyuan Ma 1 , Rui Pei 1 , Mengmei He 1 , Jian Zhou 1
|
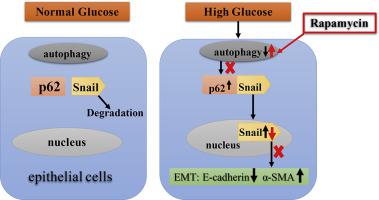
Subcapsular cataracts are common phenotype of diabetic cataracts, and abnormal lens epithelial cells (LECs) under the lens capsules have been considered to involve in the pathogenesis. Our previous studies have shown that the epithelial to mesenchymal transition (EMT), which is responsible for the LECs to lose their original polarity and tight junctions, occurs in a diabetic cataract mouse model. Autophagy is known to function in the EMT process in multiple tissues. However, the relationship between autophagy and EMT process in LECs has not yet been fully demonstrated. We found that high glucose retreatment reducing expression level of E-cadherin, an epithelial marker, but increasing that of α-smooth muscle actin (α-SMA), a mesenchymal marker, by Western blot and immunoflurence staining assays, and increased the cell migration by Transwell assay in human lens epithelial cell line HLE-B3. High glucose retreatment also led to impairment of autophagy, representing by downregulation of Beclin, LC3II/LC3I, and reducing the number of autophagosomes. Activation of autophagy by rapamycin could prevent high glucose-induced EMT. In addition, the levels of p62 and Snail were increased in high glucose-treated HLE-B3 cells, and their interactions were demonstrated by co-immunoprecipitation and immunoflurence staining, but all these changes were attenuated by application of rapamycin. These findings delineated a novel autophagy-mediated mechanism, p62 might mediate Snail underlying high glucose-induced EMT in LECs, suggesting a potential therapeutic approach for diabetic cataract by regulating autophagy.
中文翻译:

自噬的激活抑制高糖条件诱导的人晶状体上皮细胞的上皮向间充质转化过程。
囊下白内障是糖尿病性白内障的常见表型,晶状体囊下的异常晶状体上皮细胞(LEC)被认为参与了发病机制。我们之前的研究表明,导致 LEC 失去其原始极性和紧密连接的上皮间质转化 (EMT) 发生在糖尿病白内障小鼠模型中。已知自噬在多种组织的 EMT 过程中起作用。然而,LECs中自噬和EMT过程之间的关系尚未得到充分证明。我们发现,通过蛋白质印迹和免疫荧光染色试验,高糖再治疗降低了上皮标志物 E-钙粘蛋白的表达水平,但增加了间充质标志物 α-平滑肌肌动蛋白 (α-SMA) 的表达水平,并通过 Transwell 测定在人晶状体上皮细胞系 HLE-B3 中增加细胞迁移。高糖再治疗也导致自噬受损,表现为 Beclin、LC3II/LC3I 下调和自噬体数量减少。雷帕霉素激活自噬可以防止高糖诱导的 EMT。此外,高糖处理的 HLE-B3 细胞中 p62 和 Snail 的水平增加,它们的相互作用通过免疫共沉淀和免疫荧光染色得到证实,但所有这些变化都通过应用雷帕霉素减弱。这些发现描绘了一种新的自噬介导的机制,p62 可能在 LEC 中介导 Snail 潜在的高糖诱导的 EMT,表明通过调节自噬治疗糖尿病性白内障的潜在方法。高糖再治疗也导致自噬受损,表现为 Beclin、LC3II/LC3I 下调和自噬体数量减少。雷帕霉素激活自噬可以防止高糖诱导的 EMT。此外,高糖处理的 HLE-B3 细胞中 p62 和 Snail 的水平增加,它们的相互作用通过免疫共沉淀和免疫荧光染色得到证实,但所有这些变化都通过应用雷帕霉素减弱。这些发现描绘了一种新的自噬介导的机制,p62 可能在 LEC 中介导 Snail 潜在的高糖诱导的 EMT,表明通过调节自噬治疗糖尿病性白内障的潜在方法。高糖再治疗也导致自噬受损,表现为 Beclin、LC3II/LC3I 下调和自噬体数量减少。雷帕霉素激活自噬可以防止高糖诱导的 EMT。此外,高糖处理的 HLE-B3 细胞中 p62 和 Snail 的水平增加,它们的相互作用通过共免疫沉淀和免疫荧光染色得到证实,但所有这些变化都通过应用雷帕霉素减弱。这些发现描绘了一种新的自噬介导的机制,p62 可能在 LEC 中介导 Snail 潜在的高糖诱导的 EMT,表明通过调节自噬治疗糖尿病性白内障的潜在方法。并减少自噬体的数量。雷帕霉素激活自噬可以防止高糖诱导的 EMT。此外,高糖处理的 HLE-B3 细胞中 p62 和 Snail 的水平增加,它们的相互作用通过免疫共沉淀和免疫荧光染色得到证实,但所有这些变化都通过应用雷帕霉素减弱。这些发现描绘了一种新的自噬介导的机制,p62 可能在 LEC 中介导 Snail 潜在的高糖诱导的 EMT,表明通过调节自噬治疗糖尿病性白内障的潜在方法。并减少自噬体的数量。雷帕霉素激活自噬可以防止高糖诱导的 EMT。此外,高糖处理的 HLE-B3 细胞中 p62 和 Snail 的水平增加,它们的相互作用通过免疫共沉淀和免疫荧光染色得到证实,但所有这些变化都通过应用雷帕霉素减弱。这些发现描绘了一种新的自噬介导的机制,p62 可能在 LEC 中介导 Snail 潜在的高糖诱导的 EMT,表明通过调节自噬治疗糖尿病性白内障的潜在方法。免疫共沉淀和免疫荧光染色证明了它们的相互作用,但所有这些变化都通过应用雷帕霉素减弱。这些发现描绘了一种新的自噬介导的机制,p62 可能在 LEC 中介导 Snail 潜在的高糖诱导的 EMT,表明通过调节自噬治疗糖尿病性白内障的潜在方法。免疫共沉淀和免疫荧光染色证明了它们的相互作用,但所有这些变化都通过应用雷帕霉素减弱。这些发现描绘了一种新的自噬介导的机制,p62 可能在 LEC 中介导 Snail 潜在的高糖诱导的 EMT,表明通过调节自噬治疗糖尿病性白内障的潜在方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号