当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Theoretical Study of the Recently Suggested MnVII Mechanism for O-O Bond Formation in Photosystem II.
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2020-09-02 , DOI: 10.1021/acs.jpca.0c05135 Xi-Chen Li 1 , Jing Li 1 , Per E M Siegbahn 2
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2020-09-02 , DOI: 10.1021/acs.jpca.0c05135 Xi-Chen Li 1 , Jing Li 1 , Per E M Siegbahn 2
Affiliation
|
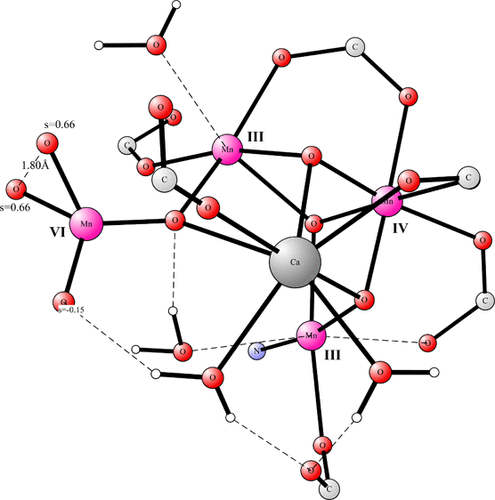
The mechanism for water oxidation in photosystem II has been a major topic for several decades. The active catalyst has four manganese atoms connected by bridging oxo bonds, in a complex termed the oxygen-evolving complex (OEC), which also includes a calcium atom. The O–O bond of oxygen is formed after absorption of four photons in a state of the OEC termed S4. There has been essential consensus that in the S4 state, all manganese atoms are in the Mn(IV) oxidation state. However, recently there has been a suggestion that one of the atoms is in the Mn(VII) state. In the present computational study, the feasibility of that proposal has been investigated. It is here shown that the mechanism involving Mn(VII) has a much higher barrier for forming O2 than the previous proposal with four Mn(IV) atoms.
中文翻译:

对最近提出的在光系统II中形成OO键的MnVII机理的理论研究。
几十年来,光系统II中水氧化的机理一直是一个主要话题。活性催化剂具有四个通过桥接氧代键连接的锰原子,该配合物称为氧释放络合物(OEC),其中还包含一个钙原子。氧气的O-O键是在四个光子被吸收后以OEC的状态S 4形成的。基本共识是,在S 4状态下,所有锰原子均处于Mn(IV)氧化态。但是,近来有人提出一个原子处于Mn(VII)状态。在当前的计算研究中,已经对该建议的可行性进行了研究。此处表明,涉及Mn(VII)的机理具有更高的形成O 2的势垒。 比以前的建议有四个Mn(IV)原子。
更新日期:2020-10-02
中文翻译:

对最近提出的在光系统II中形成OO键的MnVII机理的理论研究。
几十年来,光系统II中水氧化的机理一直是一个主要话题。活性催化剂具有四个通过桥接氧代键连接的锰原子,该配合物称为氧释放络合物(OEC),其中还包含一个钙原子。氧气的O-O键是在四个光子被吸收后以OEC的状态S 4形成的。基本共识是,在S 4状态下,所有锰原子均处于Mn(IV)氧化态。但是,近来有人提出一个原子处于Mn(VII)状态。在当前的计算研究中,已经对该建议的可行性进行了研究。此处表明,涉及Mn(VII)的机理具有更高的形成O 2的势垒。 比以前的建议有四个Mn(IV)原子。



























 京公网安备 11010802027423号
京公网安备 11010802027423号