当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Semicoordination Bond Breaking and Halogen Bond Making Change the Supramolecular Architecture of Metal-Containing Aggregates
Crystal Growth & Design ( IF 3.8 ) Pub Date : 2020-09-02 , DOI: 10.1021/acs.cgd.0c00999 Lev E. Zelenkov 1, 2 , Daniil M. Ivanov 1 , Evgeniy K. Sadykov 3 , Nadezhda A. Bokach 1 , Bartomeu Galmés 4 , Antonio Frontera 4 , Vadim Yu. Kukushkin 1, 5
Crystal Growth & Design ( IF 3.8 ) Pub Date : 2020-09-02 , DOI: 10.1021/acs.cgd.0c00999 Lev E. Zelenkov 1, 2 , Daniil M. Ivanov 1 , Evgeniy K. Sadykov 3 , Nadezhda A. Bokach 1 , Bartomeu Galmés 4 , Antonio Frontera 4 , Vadim Yu. Kukushkin 1, 5
Affiliation
|
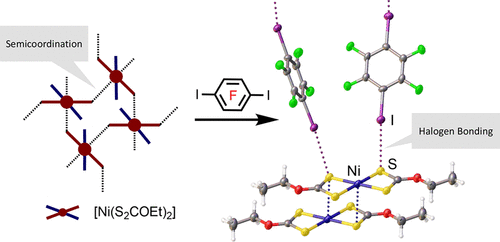
The complex [Ni(S2COEt)2] (1) and 1,4-diiodotertafluorobenzene (1,4-FIB) or 1,3,5-triiodotrifluorobenzene (1,3,5-FIB) were cocrystallized to form solid adducts 1·2(1,4-FIB) and 1·2(1,3,5-FIB), respectively; the structures of the adducts were studied by X-ray crystallography. The introduction of any one of the FIBs dramatically changed the supramolecular architecture of 1, and the structure-directing interactions changed from predominantly Ni···S semicoordination (in 1) to I···S halogen bonding between an FIB and the electron-donating S atoms of 1 (in the adducts). The semicoordination bond breaking and halogen bond making upon the interaction of 1 with the FIBs make the employed crystal engineering approach relevant (or even similar) to the molecular synthesis of metal species. The DFT study indicates that the strength of both types of interactions in the adducts are comparable (−3.0 to −4.9 kcal/mol and −4.3 to −4.9 kcal/mol) but very different in regard to their physical nature. If the electrostatics determine the I···S halogen bonds, the Ni···S semicoordination bonding is basically dominated by orbital effects.
中文翻译:

半配位键断裂和卤素键的产生改变了含金属团聚体的超分子结构
将[Ni(S 2 COEt)2 ](1)与1,4-二碘四氟苯(1,4-FIB)或1,3,5-三碘三氟苯(1,3,5-FIB)共结晶以形成固体加合物1 ·2(1,4-FIB)和1 ·2(1,3,5-FIB);通过X射线晶体学研究了加合物的结构。任何一种FIB的引入都极大地改变了1的超分子结构,并且结构导向的相互作用从主要的Ni···S半配位(在1中)变为FI·B和电子-之间的I ·· S卤素键。捐赠1个S原子(在加合物中)。1与FIB相互作用时的半配位键断裂和卤素键形成使得所采用的晶体工程方法与金属物种的分子合成相关(或什至相似)。DFT研究表明,加合物中两种类型相互作用的强度是可比的(-3.0至-4.9 kcal / mol和-4.3至-4.9 kcal / mol),但在物理性质方面却大不相同。如果静电确定I··S卤素键,则Ni··S半配位键基本上受轨道效应支配。
更新日期:2020-10-07
中文翻译:

半配位键断裂和卤素键的产生改变了含金属团聚体的超分子结构
将[Ni(S 2 COEt)2 ](1)与1,4-二碘四氟苯(1,4-FIB)或1,3,5-三碘三氟苯(1,3,5-FIB)共结晶以形成固体加合物1 ·2(1,4-FIB)和1 ·2(1,3,5-FIB);通过X射线晶体学研究了加合物的结构。任何一种FIB的引入都极大地改变了1的超分子结构,并且结构导向的相互作用从主要的Ni···S半配位(在1中)变为FI·B和电子-之间的I ·· S卤素键。捐赠1个S原子(在加合物中)。1与FIB相互作用时的半配位键断裂和卤素键形成使得所采用的晶体工程方法与金属物种的分子合成相关(或什至相似)。DFT研究表明,加合物中两种类型相互作用的强度是可比的(-3.0至-4.9 kcal / mol和-4.3至-4.9 kcal / mol),但在物理性质方面却大不相同。如果静电确定I··S卤素键,则Ni··S半配位键基本上受轨道效应支配。


























 京公网安备 11010802027423号
京公网安备 11010802027423号