当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ligand- and pH-Induced Structural Transition of Gypsy Moth Lymantria dispar Pheromone-Binding Protein 1 (LdisPBP1).
Biochemistry ( IF 2.9 ) Pub Date : 2020-09-02 , DOI: 10.1021/acs.biochem.0c00592 Mailyn Terrado 1 , Mark Okon 2 , Lawrence P McIntosh 2 , Erika Plettner 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-09-02 , DOI: 10.1021/acs.biochem.0c00592 Mailyn Terrado 1 , Mark Okon 2 , Lawrence P McIntosh 2 , Erika Plettner 1
Affiliation
|
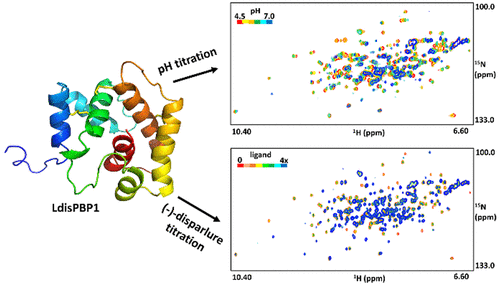
Pheromone-binding proteins (PBPs) are small, water-soluble proteins found in the lymph of pheromone-sensing hairs. PBPs are essential in modulating pheromone partitioning in the lymph and at pheromone receptors of olfactory sensory neurons. The function of a PBP is associated with its ability to structurally convert between two conformations. Although mechanistic details remain unclear, it has been proposed that the structural transition between these forms is affected by two factors: pH and the presence or absence of ligand. To better understand the PBP conformational transition, the structure of the gypsy moth (Lymantria dispar) LdisPBP1 was elucidated at pH 4.5 and 35 °C using nuclear magnetic resonance spectroscopy. In addition, the effects of sample pH and binding of the species’ pheromone, (+)-disparlure, (7R,8S)-epoxy-2-methyloctadecane, and its enantiomer were monitored via 15N HSQC spectroscopy. LdisPBP1 in acidic conditions has seven helices, with its C-terminal residues forming the seventh helix within the pheromone-binding pocket and its N-terminal residues disordered. Under conditions where this conformation is made favorable, free LdisPBP1 would have limited ligand binding capacity due to the seventh helix occupying the internal binding pocket. Our findings suggest that even in the presence of 4-fold ligand at acidic pH, LdisPBP1 is only ∼60% in its pheromone-bound form. Furthermore, evidence of a different LdisPBP1 form is seen at higher pH, with the transition pH between 5.6 and 6.0. This suggests that LdisPBP1 at neutral pH exists as a mixture of at least two conformations. These findings have implications concerning the PBP ligand binding and release mechanism.
中文翻译:

配体和pH诱导的吉普赛蛾Lymantria dispar信息素结合蛋白1(LdisPBP1)的结构转变。
信息素结合蛋白(PBP)是在信息素敏感头发的淋巴液中发现的一种小的水溶性蛋白。PBP对调节嗅觉感觉神经元的淋巴和信息素受体中的信息素分配至关重要。PBP的功能与其在两个构象之间进行结构转化的能力有关。尽管机械细节仍不清楚,但已提出这些形式之间的结构转变受两个因素影响:pH和配体的存在与否。为了更好地了解PBP构象转变,吉普赛蛾的结构(Lymantria dispar)使用核磁共振波谱法在pH 4.5和35°C下阐明了LdisPBP1。此外,通过15监测了样品pH值和物种信息素,(+)-分散,(7 R,8 S)-环氧-2-甲基十八烷及其对映体结合的影响。N HSQC光谱。在酸性条件下,LdisPBP1具有七个螺旋,其C端残基在信息素结合袋中形成第七个螺旋,其N端残基无序。在使该构象有利的条件下,由于第七螺旋占据了内部结合袋,游离的LdisPBP1将具有有限的配体结合能力。我们的发现表明,即使在酸性pH下存在4倍配体,LdisPBP1的信息素结合形式也仅为约60%。此外,在较高的pH值(过渡pH在5.6和6.0之间)时,可以看到LdisPBP1形式不同的证据。这表明在中性pH下的LdisPBP1以至少两种构象的混合物形式存在。这些发现与PBP配体的结合和释放机理有关。
更新日期:2020-09-22
中文翻译:

配体和pH诱导的吉普赛蛾Lymantria dispar信息素结合蛋白1(LdisPBP1)的结构转变。
信息素结合蛋白(PBP)是在信息素敏感头发的淋巴液中发现的一种小的水溶性蛋白。PBP对调节嗅觉感觉神经元的淋巴和信息素受体中的信息素分配至关重要。PBP的功能与其在两个构象之间进行结构转化的能力有关。尽管机械细节仍不清楚,但已提出这些形式之间的结构转变受两个因素影响:pH和配体的存在与否。为了更好地了解PBP构象转变,吉普赛蛾的结构(Lymantria dispar)使用核磁共振波谱法在pH 4.5和35°C下阐明了LdisPBP1。此外,通过15监测了样品pH值和物种信息素,(+)-分散,(7 R,8 S)-环氧-2-甲基十八烷及其对映体结合的影响。N HSQC光谱。在酸性条件下,LdisPBP1具有七个螺旋,其C端残基在信息素结合袋中形成第七个螺旋,其N端残基无序。在使该构象有利的条件下,由于第七螺旋占据了内部结合袋,游离的LdisPBP1将具有有限的配体结合能力。我们的发现表明,即使在酸性pH下存在4倍配体,LdisPBP1的信息素结合形式也仅为约60%。此外,在较高的pH值(过渡pH在5.6和6.0之间)时,可以看到LdisPBP1形式不同的证据。这表明在中性pH下的LdisPBP1以至少两种构象的混合物形式存在。这些发现与PBP配体的结合和释放机理有关。


























 京公网安备 11010802027423号
京公网安备 11010802027423号