当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Regioselectivity of the trifluoroethanol-promoted intramolecular N-Boc-epoxide cyclization towards 1,3-oxazolidin-2-ones and 1,3-oxazinan-2-ones.
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-09-02 , DOI: 10.1039/d0ob01698e Hemi Borgohain 1 , Kangkan Talukdar 1 , Bipul Sarma 1 , Sajal Kumar Das 1
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-09-02 , DOI: 10.1039/d0ob01698e Hemi Borgohain 1 , Kangkan Talukdar 1 , Bipul Sarma 1 , Sajal Kumar Das 1
Affiliation
|
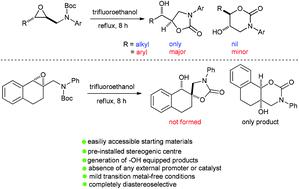
The intramolecular N-Boc–epoxide cyclization leading to the formation of 1,3-oxazolidin-2-one and 1,3-oxazinan-2-one derivatives has scarcely been reported in the literature. More specifically, the intramolecular cyclization of N-Boc aniline-tethered 2,3-disubstitued epoxides has never been disclosed. Herein, we demonstrate that this reaction could proceed in a diastereoselective fashion in refluxing trifluoroethanol, in the absence of any external promoter or catalyst. Substrates bearing an alkyl group at the C-3 position furnished 1,3-oxazolidin-2-ones in a completely regioselective fashion via 5-exo epoxide ring-opening cyclization, thereby paving the way to synthesize alkyl side chain-bearing analogs of the antidepressant drug toloxatone. On the other hand, replacing the alkyl group with an aryl group resulted in easily separable mixtures of 1,3-oxazolidin-2-ones and 1,3-oxazinan-2-ones, the former being obtained as the major products. Remarkably, a tetralin-bearing substrate underwent fully regioselective 6-endo ring closure to form the corresponding 1,3-oxazinan-2-one. Our present study on the intramolecular ring opening-cyclization of epoxides with a tethered N-Boc group is the most comprehensive to date and features broad substrate scope, mild transition metal-free conditions, excellent functional group tolerance, and scalability.
中文翻译:

三氟乙醇促进的分子内 N-Boc-环氧化物环化对 1,3-oxazolidin-2-ones 和 1,3-oxazinan-2-ones 的区域选择性。
导致形成 1,3-oxazolidin-2-one 和 1,3-oxazinan-2-one 衍生物的分子内N -Boc-环氧化物环化在文献中几乎没有报道。更具体地说,从未公开过N -Boc 苯胺连接的 2,3-二取代环氧化物的分子内环化。在此,我们证明了在没有任何外部促进剂或催化剂的情况下,该反应可以在回流的三氟乙醇中以非对映选择性方式进行。在 C-3 位带有烷基的底物通过5 - exo以完全区域选择性的方式提供 1,3-oxazolidin-2-ones环氧化物开环环化,从而为合成抗抑郁药托洛沙酮的烷基侧链类似物铺平了道路。另一方面,用芳基取代烷基导致1,3-恶唑烷-2-酮和1,3-恶唑烷-2-酮的易于分离的混合物,前者作为主要产物获得。值得注意的是,带有四氢化萘的底物经历了完全区域选择性的 6-内环闭合形成相应的 1,3-oxazinan-2-one 。我们目前对具有束缚N - Boc基团的环氧化物的分子内开环环化的研究是迄今为止最全面的,并且具有广泛的底物范围、温和的无过渡金属条件、优异的官能团耐受性和可扩展性。
更新日期:2020-09-30
中文翻译:

三氟乙醇促进的分子内 N-Boc-环氧化物环化对 1,3-oxazolidin-2-ones 和 1,3-oxazinan-2-ones 的区域选择性。
导致形成 1,3-oxazolidin-2-one 和 1,3-oxazinan-2-one 衍生物的分子内N -Boc-环氧化物环化在文献中几乎没有报道。更具体地说,从未公开过N -Boc 苯胺连接的 2,3-二取代环氧化物的分子内环化。在此,我们证明了在没有任何外部促进剂或催化剂的情况下,该反应可以在回流的三氟乙醇中以非对映选择性方式进行。在 C-3 位带有烷基的底物通过5 - exo以完全区域选择性的方式提供 1,3-oxazolidin-2-ones环氧化物开环环化,从而为合成抗抑郁药托洛沙酮的烷基侧链类似物铺平了道路。另一方面,用芳基取代烷基导致1,3-恶唑烷-2-酮和1,3-恶唑烷-2-酮的易于分离的混合物,前者作为主要产物获得。值得注意的是,带有四氢化萘的底物经历了完全区域选择性的 6-内环闭合形成相应的 1,3-oxazinan-2-one 。我们目前对具有束缚N - Boc基团的环氧化物的分子内开环环化的研究是迄今为止最全面的,并且具有广泛的底物范围、温和的无过渡金属条件、优异的官能团耐受性和可扩展性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号