当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Pico-meter resolution structure of the coordination sphere in the metal-binding site in a metalloprotein by NMR
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-09-01 , DOI: 10.1021/jacs.0c07339 Andrea Bertarello 1, 2 , Ladislav Benda 1 , Kevin J Sanders 1 , Andrew J Pell 1 , Michael J Knight 1 , Vladimir Pelmenschikov 3 , Leonardo Gonnelli 4 , Isabella C Felli 4 , Martin Kaupp 3 , Lyndon Emsley 2 , Roberta Pierattelli 4 , Guido Pintacuda 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-09-01 , DOI: 10.1021/jacs.0c07339 Andrea Bertarello 1, 2 , Ladislav Benda 1 , Kevin J Sanders 1 , Andrew J Pell 1 , Michael J Knight 1 , Vladimir Pelmenschikov 3 , Leonardo Gonnelli 4 , Isabella C Felli 4 , Martin Kaupp 3 , Lyndon Emsley 2 , Roberta Pierattelli 4 , Guido Pintacuda 1
Affiliation
|
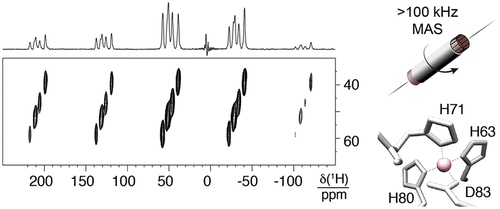
Most of our understanding of chemistry derives from atomic-level structures obtained with single crystal X-ray diffraction. Metal centers in X-ray structures of small organometallic or coordination complexes are often extremely well defined, with errors in the positions on the order of 10-4-10-5 Å. Determining the metal coordination geometry to high accuracy is essential for understanding metal center reactivity, as even small structural changes can dramatically alter the metal activity. In contrast, the resolution of X-ray structures in proteins is limited typically to the order of 10-1 Å. This resolution is often not sufficient to develop precise structure-activity relations for the metal sites in proteins, since the uncertainty in positions can cover all the known ranges of bond-lengths and bond-angles for a given type of metal-complex. Here we introduce a new approach that enables determination of a high-definition structure of the active site of a metalloprotein from a powder sample, by combining magic-angle spinning (MAS) nuclear magnetic resonance (NMR) spectroscopy, tailored radio-frequency (RF) irradiation schemes, and computational approaches. This allows us to overcome the "blind sphere" in paramagnetic proteins, and to observe and assign 1H, 13C, and 15N resonances for the ligands directly coordinating the metal center. We illustrate the method by determining the bond lengths in the structure of the CoII coordination sphere at the core of human superoxide dismutase 1 (SOD) with 0.7 pm precision. The coordination geometry of the resulting structure explains the non-reactive nature of the CoII/ZnII centers in these proteins, that allows them to play a purely structural role.
中文翻译:

核磁共振金属蛋白金属结合位点配位球的皮米分辨率结构
我们对化学的大部分理解来自于通过单晶 X 射线衍射获得的原子级结构。小的有机金属或配位配合物的 X 射线结构中的金属中心通常非常明确,位置误差约为 10-4-10-5 Å。以高精度确定金属配位几何对于理解金属中心反应性至关重要,因为即使是很小的结构变化也会显着改变金属活性。相比之下,蛋白质中 X 射线结构的分辨率通常限制在 10-1 Å 的数量级。这种分辨率通常不足以为蛋白质中的金属位点建立精确的构效关系,因为位置的不确定性可以涵盖给定类型的金属复合物的所有已知的键长和键角范围。在这里,我们介绍了一种新方法,通过结合魔角旋转 (MAS) 核磁共振 (NMR) 光谱、定制射频 (RF ) 辐照方案和计算方法。这使我们能够克服顺磁性蛋白质中的“盲球”,并观察和分配直接协调金属中心的配体的 1H、13C 和 15N 共振。我们通过确定人类超氧化物歧化酶 1 (SOD) 核心 CoII 配位球结构中的键长来说明该方法,精度为 0.7 pm。所得结构的配位几何解释了这些蛋白质中 CoII/ZnII 中心的非反应性,
更新日期:2020-09-01
中文翻译:

核磁共振金属蛋白金属结合位点配位球的皮米分辨率结构
我们对化学的大部分理解来自于通过单晶 X 射线衍射获得的原子级结构。小的有机金属或配位配合物的 X 射线结构中的金属中心通常非常明确,位置误差约为 10-4-10-5 Å。以高精度确定金属配位几何对于理解金属中心反应性至关重要,因为即使是很小的结构变化也会显着改变金属活性。相比之下,蛋白质中 X 射线结构的分辨率通常限制在 10-1 Å 的数量级。这种分辨率通常不足以为蛋白质中的金属位点建立精确的构效关系,因为位置的不确定性可以涵盖给定类型的金属复合物的所有已知的键长和键角范围。在这里,我们介绍了一种新方法,通过结合魔角旋转 (MAS) 核磁共振 (NMR) 光谱、定制射频 (RF ) 辐照方案和计算方法。这使我们能够克服顺磁性蛋白质中的“盲球”,并观察和分配直接协调金属中心的配体的 1H、13C 和 15N 共振。我们通过确定人类超氧化物歧化酶 1 (SOD) 核心 CoII 配位球结构中的键长来说明该方法,精度为 0.7 pm。所得结构的配位几何解释了这些蛋白质中 CoII/ZnII 中心的非反应性,



























 京公网安备 11010802027423号
京公网安备 11010802027423号