当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Potential Doxorubicin-Mediated Dual-Targeting Chemotherapy in FANC/BRCA-Deficient Tumors via Modulation of Cellular Formaldehyde Concentration.
Chemical Research in Toxicology ( IF 4.1 ) Pub Date : 2020-09-02 , DOI: 10.1021/acs.chemrestox.0c00288 Jun Nakamura 1, 2
Chemical Research in Toxicology ( IF 4.1 ) Pub Date : 2020-09-02 , DOI: 10.1021/acs.chemrestox.0c00288 Jun Nakamura 1, 2
Affiliation
|
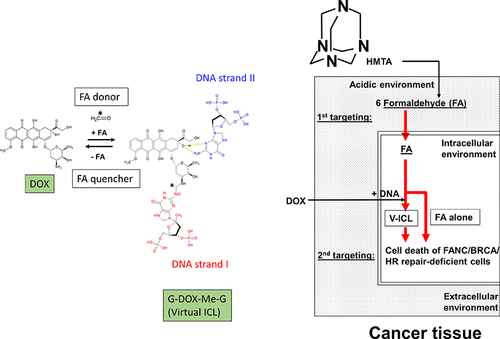
Doxorubicin (DOX) is a widely used classical broad-spectrum anticancer drug. The major mechanism of DOX-mediated anticancer activity at clinically relevant concentrations is believed to be via DNA double-strand breaks due to topoisomerase IIα. However, other mechanisms by which DOX causes cytotoxicity have been proposed, including formaldehyde-dependent virtual interstrand cross-linking (ICL) formation. In this study, a method was established whereby cytotoxicity caused by virtual ICL derived from DOX is turned on and off using a cell culture system. Using this strategy, DOX-mediated cytotoxicity in Fanconi anemia group gene (FANC)/breast cancer susceptibility gene (BRCA)-deficient cells increased up to 70-fold compared to that in cells proficient in DNA repair pathways by increasing intracellular formaldehyde (FA) concentration. This approach also demonstrated that cytotoxicity introduced by DOX-mediated FA-dependent virtual ICL is completely independent of the toxicity induced by topoisomerase II inhibition at the cellular level. The potential of dual-targeting by DOX treatment was verified using an acid-specific FA donor. Overall, anticancer therapy targeting tumors deficient in the FANC/BRCA pathway may be possible by minimizing DOX-induced toxicity in normal cells.
中文翻译:

通过调节细胞甲醛浓度在 FANC/BRCA 缺陷肿瘤中进行潜在的阿霉素介导的双靶向化疗。
多柔比星(DOX)是一种广泛使用的经典广谱抗癌药物。在临床相关浓度下,DOX 介导的抗癌活性的主要机制被认为是通过拓扑异构酶 IIα 引起的 DNA 双链断裂。然而,已经提出了 DOX 引起细胞毒性的其他机制,包括甲醛依赖性虚拟链间交联 (ICL) 的形成。在这项研究中,建立了一种方法,即使用细胞培养系统打开和关闭由来自 DOX 的虚拟 ICL 引起的细胞毒性。使用这种策略,DOX 介导的范可尼贫血组基因 ( FANC )/乳腺癌易感基因 ( BRCA ) 中的细胞毒性)-缺陷细胞通过增加细胞内甲醛 (FA) 浓度与精通 DNA 修复途径的细胞相比增加了 70 倍。这种方法还表明,由 DOX 介导的 FA 依赖性虚拟 ICL 引入的细胞毒性完全独立于细胞水平的拓扑异构酶 II 抑制诱导的毒性。使用酸特异性 FA 供体验证了 DOX 处理双重靶向的潜力。总体而言,通过最大限度地减少 DOX 诱导的正常细胞毒性,靶向 FANC/BRCA 通路缺陷肿瘤的抗癌治疗可能成为可能。
更新日期:2020-10-21
中文翻译:

通过调节细胞甲醛浓度在 FANC/BRCA 缺陷肿瘤中进行潜在的阿霉素介导的双靶向化疗。
多柔比星(DOX)是一种广泛使用的经典广谱抗癌药物。在临床相关浓度下,DOX 介导的抗癌活性的主要机制被认为是通过拓扑异构酶 IIα 引起的 DNA 双链断裂。然而,已经提出了 DOX 引起细胞毒性的其他机制,包括甲醛依赖性虚拟链间交联 (ICL) 的形成。在这项研究中,建立了一种方法,即使用细胞培养系统打开和关闭由来自 DOX 的虚拟 ICL 引起的细胞毒性。使用这种策略,DOX 介导的范可尼贫血组基因 ( FANC )/乳腺癌易感基因 ( BRCA ) 中的细胞毒性)-缺陷细胞通过增加细胞内甲醛 (FA) 浓度与精通 DNA 修复途径的细胞相比增加了 70 倍。这种方法还表明,由 DOX 介导的 FA 依赖性虚拟 ICL 引入的细胞毒性完全独立于细胞水平的拓扑异构酶 II 抑制诱导的毒性。使用酸特异性 FA 供体验证了 DOX 处理双重靶向的潜力。总体而言,通过最大限度地减少 DOX 诱导的正常细胞毒性,靶向 FANC/BRCA 通路缺陷肿瘤的抗癌治疗可能成为可能。



























 京公网安备 11010802027423号
京公网安备 11010802027423号