当前位置:
X-MOL 学术
›
ACS Pharmacol. Transl. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ligand-Specific Allosteric Coupling Controls G-Protein-Coupled Receptor Signaling
ACS Pharmacology & Translational Science Pub Date : 2020-09-02 , DOI: 10.1021/acsptsci.0c00069 Janine Holze 1 , Marcel Bermudez 2 , Eva Marie Pfeil 3 , Michael Kauk 4 , Theresa Bödefeld 1 , Matthias Irmen 1 , Carlo Matera 5 , Clelia Dallanoce 5 , Marco De Amici 5 , Ulrike Holzgrabe 6 , Gabriele Maria König 7 , Christian Tränkle 1 , Gerhard Wolber 2 , Ramona Schrage 1 , Klaus Mohr 1 , Carsten Hoffmann 4 , Evi Kostenis 3 , Andreas Bock 1, 8
ACS Pharmacology & Translational Science Pub Date : 2020-09-02 , DOI: 10.1021/acsptsci.0c00069 Janine Holze 1 , Marcel Bermudez 2 , Eva Marie Pfeil 3 , Michael Kauk 4 , Theresa Bödefeld 1 , Matthias Irmen 1 , Carlo Matera 5 , Clelia Dallanoce 5 , Marco De Amici 5 , Ulrike Holzgrabe 6 , Gabriele Maria König 7 , Christian Tränkle 1 , Gerhard Wolber 2 , Ramona Schrage 1 , Klaus Mohr 1 , Carsten Hoffmann 4 , Evi Kostenis 3 , Andreas Bock 1, 8
Affiliation
|
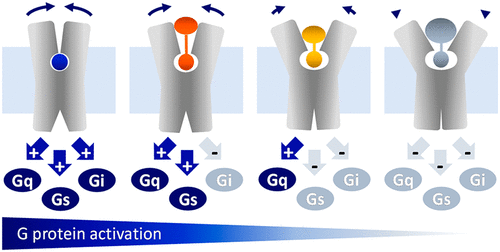
Allosteric coupling describes a reciprocal process whereby G-protein-coupled receptors (GPCRs) relay ligand-induced conformational changes from the extracellular binding pocket to the intracellular signaling surface. Therefore, GPCR activation is sensitive to both the type of extracellular ligand and intracellular signaling protein. We hypothesized that ligand-specific allosteric coupling may result in preferential (i.e., biased) engagement of downstream effectors. However, the structural basis underlying ligand-dependent control of this essential allosteric mechanism is poorly understood. Here, we show that two sets of extended muscarinic acetylcholine receptor M1 agonists, which only differ in linker length, progressively constrain receptor signaling. We demonstrate that stepwise shortening of their chemical linker gradually hampers binding pocket closure, resulting in divergent coupling to distinct G-protein families. Our data provide an experimental strategy for the design of ligands with selective G-protein recognition and reveal a potentially general mechanism of ligand-specific allosteric coupling.
中文翻译:

配体特异性变构偶联控制 G 蛋白偶联受体信号
别构耦合描述了一个互惠过程,其中 G 蛋白偶联受体 (GPCR) 将配体诱导的构象变化从细胞外结合口袋传递到细胞内信号传导表面。因此,GPCR 激活对细胞外配体和细胞内信号蛋白的类型都很敏感。我们假设配体特异性变构偶联可能导致下游效应器的优先(即,有偏差)参与。然而,对这种基本变构机制的配体依赖性控制的结构基础知之甚少。在这里,我们展示了两组扩展的毒蕈碱乙酰胆碱受体 M 1仅在接头长度上不同的激动剂逐渐限制受体信号传导。我们证明逐步缩短其化学接头会逐渐阻碍结合口袋闭合,从而导致与不同 G 蛋白家族的不同耦合。我们的数据为设计具有选择性 G 蛋白识别的配体提供了一种实验策略,并揭示了配体特异性变构偶联的潜在通用机制。
更新日期:2020-10-11
中文翻译:

配体特异性变构偶联控制 G 蛋白偶联受体信号
别构耦合描述了一个互惠过程,其中 G 蛋白偶联受体 (GPCR) 将配体诱导的构象变化从细胞外结合口袋传递到细胞内信号传导表面。因此,GPCR 激活对细胞外配体和细胞内信号蛋白的类型都很敏感。我们假设配体特异性变构偶联可能导致下游效应器的优先(即,有偏差)参与。然而,对这种基本变构机制的配体依赖性控制的结构基础知之甚少。在这里,我们展示了两组扩展的毒蕈碱乙酰胆碱受体 M 1仅在接头长度上不同的激动剂逐渐限制受体信号传导。我们证明逐步缩短其化学接头会逐渐阻碍结合口袋闭合,从而导致与不同 G 蛋白家族的不同耦合。我们的数据为设计具有选择性 G 蛋白识别的配体提供了一种实验策略,并揭示了配体特异性变构偶联的潜在通用机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号