当前位置:
X-MOL 学术
›
Fluid Phase Equilibr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Insights into the Structural and Thermodynamic Properties of Fullerols [C60(OH)n, n = 12, 14, 16, 18, 20, 22, 24] in Aqueous Media
Fluid Phase Equilibria ( IF 2.6 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.fluid.2020.112805 Sonanki Keshri
Fluid Phase Equilibria ( IF 2.6 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.fluid.2020.112805 Sonanki Keshri
|
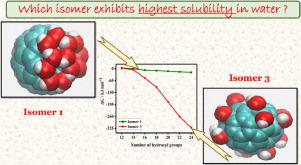
Abstract In recent years, fullerenes and their amphiphilic derivatives fullerols [C60(OH)n] have received considerable attention of researchers worldwide because of their unique structural and electronic properties that enables numerous industrial and medicinal applications. Because of surface hydroxylation in fullerols [C60(OH)n], one might expect a different behaviour of these hydrophobic-hydrophilic solutes in water. In the present study, we have performed classical molecular dynamics simulations of three different isomers of fullerols [C60(OH)n, where n = 12-24]. Hydration shells of water around these nanoparticles are characterized with the help of radial distribution functions (RDFs) and hydrogen bonding. RDFs indicate the presence of two hydration shells around the central solute molecule for all the isomers. Hydrogen bonding studies reveal that with uniform distribution of hydroxyl groups on fullerene cage, the average number of solute-solvent hydrogen bond increases with an increasing number of hydroxyl groups. The hydration free energy for the isomer with uniform distribution of hydroxyl group is found to be highly negative compared to all other isomers for all the fullerols, which suggests that the effect of surface hydroxylation is more dominant where the hydroxyl groups are uniformly distributed on the fullerene cage.
中文翻译:

深入了解富勒醇 [C60(OH)n, n = 12, 14, 16, 18, 20, 22, 24] 在水性介质中的结构和热力学特性
摘要 近年来,富勒烯及其两亲性衍生物富勒醇 [C60(OH)n] 因其独特的结构和电子特性而受到全世界研究人员的广泛关注,使其能够实现众多工业和医学应用。由于富勒醇 [C60(OH)n] 中的表面羟基化,人们可能会预期这些疏水-亲水溶质在水中的行为会有所不同。在本研究中,我们对富勒醇的三种不同异构体 [C60(OH)n,其中 n = 12-24] 进行了经典分子动力学模拟。这些纳米粒子周围的水化壳借助径向分布函数 (RDF) 和氢键进行表征。RDF 表明所有异构体的中心溶质分子周围都存在两个水合壳。氢键研究表明,随着羟基在富勒烯笼上的均匀分布,溶质-溶剂氢键的平均数量随着羟基数量的增加而增加。发现与所有富勒醇的所有其他异构体相比,具有均匀分布的羟基的异构体的水合自由能高度为负,这表明表面羟基化的影响更为显着,其中羟基均匀分布在富勒烯上笼。
更新日期:2020-12-01
中文翻译:

深入了解富勒醇 [C60(OH)n, n = 12, 14, 16, 18, 20, 22, 24] 在水性介质中的结构和热力学特性
摘要 近年来,富勒烯及其两亲性衍生物富勒醇 [C60(OH)n] 因其独特的结构和电子特性而受到全世界研究人员的广泛关注,使其能够实现众多工业和医学应用。由于富勒醇 [C60(OH)n] 中的表面羟基化,人们可能会预期这些疏水-亲水溶质在水中的行为会有所不同。在本研究中,我们对富勒醇的三种不同异构体 [C60(OH)n,其中 n = 12-24] 进行了经典分子动力学模拟。这些纳米粒子周围的水化壳借助径向分布函数 (RDF) 和氢键进行表征。RDF 表明所有异构体的中心溶质分子周围都存在两个水合壳。氢键研究表明,随着羟基在富勒烯笼上的均匀分布,溶质-溶剂氢键的平均数量随着羟基数量的增加而增加。发现与所有富勒醇的所有其他异构体相比,具有均匀分布的羟基的异构体的水合自由能高度为负,这表明表面羟基化的影响更为显着,其中羟基均匀分布在富勒烯上笼。


























 京公网安备 11010802027423号
京公网安备 11010802027423号