当前位置:
X-MOL 学术
›
Desalination
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Elucidation of the desalination mechanism of solvent extraction method through molecular modeling studies
Desalination ( IF 9.9 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.desal.2020.114704 Minsup Kim , Oh Kyung Choi , Yeongrae Cho , Jae Woo Lee , Art E. Cho
Desalination ( IF 9.9 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.desal.2020.114704 Minsup Kim , Oh Kyung Choi , Yeongrae Cho , Jae Woo Lee , Art E. Cho
|
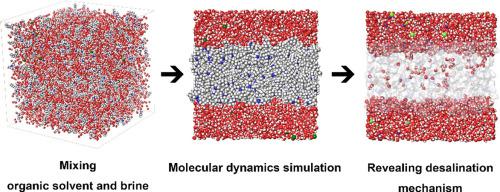
Abstract Desalination by solvent extraction is a simple and cost-effective technology to replace current ones. Although desalination solvents are continually developed, there is still a lack of understanding of the mechanism. In this study, we elucidate the solvent extraction desalination mechanism at the atomic level using molecular dynamics(MD) simulations. Three organic solvents octylamine(8A), dibutylamine(DBA), and 2-ethylhexylamine(EHA), which all have the same chemical composition but the amine group at different positions, were used. We simulated the desalination process from the brine-and-organic-solvents-mixing step to the brine-separation step. The MD simulations showed that DBA and EHA formed planar clusters while 8A formed gel clusters. By analyzing MD trajectories, we identified two types of water recovery mechanism, in which DBA and EHA clusters selectively absorb water and 8A traps brine by forming a gel structure. The polar interactions between water molecules and solvents are important driving forces for the absorption of water. To explain the desalination performances of DBA and EHA, morphological characteristics and surface polarity of the clusters were measured. It was found that a higher surface polarity facilitates more water absorption. Partial recovery of the salt ions was attributed to the surface polarity of the organic solvent clusters.
中文翻译:

通过分子模型研究阐明溶剂萃取法的脱盐机理
摘要 溶剂萃取脱盐是一种替代现有技术的简单、经济的技术。尽管海水淡化溶剂在不断发展,但对其机理仍缺乏了解。在这项研究中,我们使用分子动力学(MD)模拟在原子水平上阐明了溶剂萃取脱盐机理。使用三种有机溶剂辛胺(8A)、二丁胺(DBA)和2-乙基己胺(EHA),它们都具有相同的化学组成但胺基在不同位置。我们模拟了从盐水和有机溶剂混合步骤到盐水分离步骤的脱盐过程。MD 模拟表明 DBA 和 EHA 形成平面簇,而 8A 形成凝胶簇。通过分析 MD 轨迹,我们确定了两种类型的水回收机制,其中 DBA 和 EHA 簇选择性吸收水,8A 通过形成凝胶结构捕获盐水。水分子与溶剂之间的极性相互作用是吸水的重要驱动力。为了解释 DBA 和 EHA 的脱盐性能,测量了簇的形态特征和表面极性。发现较高的表面极性有利于更多的吸水。盐离子的部分回收归因于有机溶剂簇的表面极性。测量了簇的形态特征和表面极性。发现较高的表面极性有利于更多的吸水。盐离子的部分回收归因于有机溶剂簇的表面极性。测量了簇的形态特征和表面极性。发现较高的表面极性有利于更多的吸水。盐离子的部分回收归因于有机溶剂簇的表面极性。
更新日期:2020-12-01
中文翻译:

通过分子模型研究阐明溶剂萃取法的脱盐机理
摘要 溶剂萃取脱盐是一种替代现有技术的简单、经济的技术。尽管海水淡化溶剂在不断发展,但对其机理仍缺乏了解。在这项研究中,我们使用分子动力学(MD)模拟在原子水平上阐明了溶剂萃取脱盐机理。使用三种有机溶剂辛胺(8A)、二丁胺(DBA)和2-乙基己胺(EHA),它们都具有相同的化学组成但胺基在不同位置。我们模拟了从盐水和有机溶剂混合步骤到盐水分离步骤的脱盐过程。MD 模拟表明 DBA 和 EHA 形成平面簇,而 8A 形成凝胶簇。通过分析 MD 轨迹,我们确定了两种类型的水回收机制,其中 DBA 和 EHA 簇选择性吸收水,8A 通过形成凝胶结构捕获盐水。水分子与溶剂之间的极性相互作用是吸水的重要驱动力。为了解释 DBA 和 EHA 的脱盐性能,测量了簇的形态特征和表面极性。发现较高的表面极性有利于更多的吸水。盐离子的部分回收归因于有机溶剂簇的表面极性。测量了簇的形态特征和表面极性。发现较高的表面极性有利于更多的吸水。盐离子的部分回收归因于有机溶剂簇的表面极性。测量了簇的形态特征和表面极性。发现较高的表面极性有利于更多的吸水。盐离子的部分回收归因于有机溶剂簇的表面极性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号