Chemical Physics Letters ( IF 2.8 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.cplett.2020.137946 Mirosław Jabłoński
|
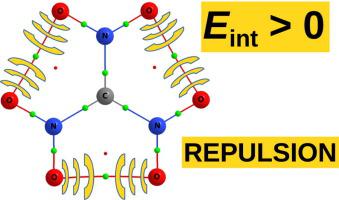
Based on the finding that, like the planar form, also the relaxed form of the ion features OO bond paths between adjacent nitro groups, Luaña et al. [J. Phys. Chem. B 107 (2003) 4912] have announced that these interactions are stabilizing. However, it will be shown that the presence of a bond path strongly depends on the computational model used and that these interactions are destabilizing. Thus the presence of a bond path between a pair of atoms is not evidence of attractive interaction between these atoms. The benefit of the partial rotation of the nitro groups during relaxation of the planar form is also given.
中文翻译:

违反直觉的结合路径:一个有趣的例子 离子
根据发现,像平面形式一样, 离子特征O相邻硝基之间的O键路径,Luaña等。[J. 物理 化学 B 107(2003)4912]宣布这些相互作用正在稳定。然而,将表明,键合路径的存在在很大程度上取决于所使用的计算模型,并且这些相互作用是不稳定的。因此,一对原子之间存在键路径并不表示这些原子之间存在吸引作用。还给出了在平面形式松弛期间硝基部分旋转的益处。



























 京公网安备 11010802027423号
京公网安备 11010802027423号