Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2020-09-02 , DOI: 10.1016/j.bioorg.2020.104254 Damian Kułaga 1 , Jolanta Jaśkowska 1 , Grzegorz Satała 2 , Gniewomir Latacz 3 , Paweł Śliwa 1
|
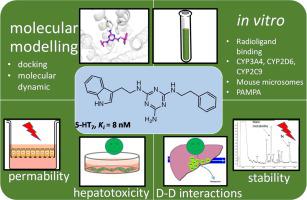
Developing new and selective 5-HT7R ligands may have a key impact on the treatment of central nervous system diseases including depression. We have found that indoleaminotriazine core fused with alkyl aryl moiety exhibits high affinity and selectivity to 5-HT7R. SAR analysis demonstrated that the ethyl or ethoxy group (5c 5-HT7R Ki = 8 nM; 5d 5-HT7R Ki = 55 nM) is the optimal carbon linker between triazine and aryl moiety. The results of the molecular dynamics simulations show stable interaction with E7.34 upon binding to a 5-HT7R. Compounds 5c and 5d were tested for early ADMET parameters. Compounds are not hepatotoxic and exhibit moderate potential interaction with other drugs metabolized by CYP3A4 or CYP2D6.
中文翻译:

具有吲哚基序的氨基三嗪为具有非典型结合模式的新型5-HT7受体配体。
开发新的选择性5-HT 7 R配体可能对中枢神经系统疾病(包括抑郁症)的治疗产生关键影响。我们发现与烷基芳基部分稠合的吲哚氨基三嗪核心对5-HT 7 R表现出高亲和力和选择性.SAR分析表明,乙基或乙氧基(5c 5-HT 7 R K i = 8 nM; 5d 5-HT 7 R K i = 55 nM)是三嗪和芳基部分之间的最佳碳连接基。分子动力学模拟的结果表明,与5-HT 7 R结合后,与E7.34稳定相互作用。化合物5c和测试5d的早期ADMET参数。该化合物无肝毒性,与其他经由CYP3A4或CYP2D6代谢的药物具有中等潜在的相互作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号