当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Access to Cyanoimines Enabled by Dual Photoredox/Copper-Catalyzed Cyanation of O-Acyl Oximes.
Organic Letters ( IF 5.2 ) Pub Date : 2020-08-31 , DOI: 10.1021/acs.orglett.0c02659 Hao Zhang 1 , Ziyan Wei 2 , Ai Hua Zhang 2 , Shouyun Yu 1
Organic Letters ( IF 5.2 ) Pub Date : 2020-08-31 , DOI: 10.1021/acs.orglett.0c02659 Hao Zhang 1 , Ziyan Wei 2 , Ai Hua Zhang 2 , Shouyun Yu 1
Affiliation
|
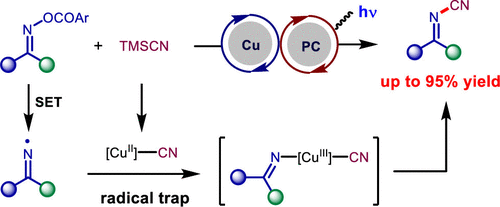
An efficient strategy for the synthesis of pharmaceutically important and synthetically useful cyanoimines, as well as cyanamides, has been described. This strategy is enabled by dual photoredox/copper-catalyzed cyanation of O-acyl oximes or O-acyl hydroxamides. This state of the art protocol for cyanoimines and cyanamides features readily available starting materials, mild reaction conditions, good functional group tolerance, and operational simplicity. The resultant cyanoimines can be transformed into structurally diverse and functionally important N-containing heterocycles.
中文翻译:

可通过O-酰基肟的双光氧化还原/铜催化氰化作用获得氰基亚胺。
已经描述了合成药学上重要的和合成上有用的氰胺以及氰胺的有效策略。该策略通过O-酰基肟或O-酰基羟酰胺的双光催化还原/铜催化的氰化作用来实现。氰胺和氰胺的这种最新技术方案具有易于获得的原料,温和的反应条件,良好的官能团耐受性和操作简便性的特点。将所得cyanoimines可以转化为结构上不同的和功能上重要的Ñ含杂环。
更新日期:2020-09-20
中文翻译:

可通过O-酰基肟的双光氧化还原/铜催化氰化作用获得氰基亚胺。
已经描述了合成药学上重要的和合成上有用的氰胺以及氰胺的有效策略。该策略通过O-酰基肟或O-酰基羟酰胺的双光催化还原/铜催化的氰化作用来实现。氰胺和氰胺的这种最新技术方案具有易于获得的原料,温和的反应条件,良好的官能团耐受性和操作简便性的特点。将所得cyanoimines可以转化为结构上不同的和功能上重要的Ñ含杂环。



























 京公网安备 11010802027423号
京公网安备 11010802027423号