当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Prediction of Crystal–Solvent Interactions in a Supercritical Medium: A Possible Way to Control Crystal Habit at High Supersaturations with Molecular Modeling
Crystal Growth & Design ( IF 3.8 ) Pub Date : 2020-08-31 , DOI: 10.1021/acs.cgd.0c00920 Sébastien Clercq 1 , Adil Mouahid 1 , Gérard Pèpe 2 , Elisabeth Badens 1
Crystal Growth & Design ( IF 3.8 ) Pub Date : 2020-08-31 , DOI: 10.1021/acs.cgd.0c00920 Sébastien Clercq 1 , Adil Mouahid 1 , Gérard Pèpe 2 , Elisabeth Badens 1
Affiliation
|
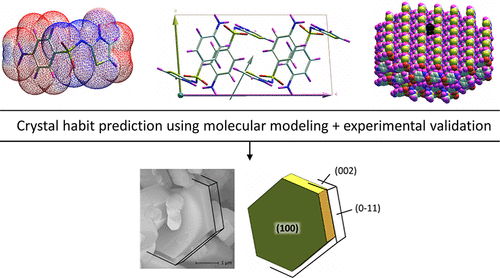
The purpose of this work is to contribute to a better control of the crystallization process which occurs in a supercritical medium, especially during the Supercritical AntiSolvent (SAS) process. It also aims to improve the prediction of crystal habit, thanks to the use of the molecular modeling software GenMol. The first part of the work was devoted to the crystal modeling of the two main forms of sulfathiazole in vacuo, considering Hartman’s attachment energy formalism. The second part considers solvent–crystal interactions throughout adsorption simulations to investigate the effect of growth environments on crystal habits. Lastly, modeling predictions were compared with grown crystals of sulfathiazole, observed after recrystallization with the SAS process from acetonitrile, acetone, tetrahydrofuran and acetic acid solutions. These comparisons demonstrated good predictions of crystal habit taking into consideration the growth environment. Neither carbon dioxide (antisolvent of the SAS process) nor acetonitrile leads to a modification of the isometric, in vacuo predicted habit of both forms. Acetone and tetrahydrofuran adsorb preferentially on some identified faces and lead to flat, leaflike, or tabular crystals. Acetic acid adsorbs on every one of the faces and hinders the phase transition to a more stable form, thus leading to crystals of the least stable, kinetically favored form I. Experimental observations were also rationalized by considering the different possible crystallization pathways, in particular Crystallization by Particle Attachment and Droplet Drying mechanisms occurring in the SAS process. This work confirms that solvent nature is one of the key elements to consider in order to better control the characteristics of particles grown using the SAS process and provides a new method to help to control it.
中文翻译:

超临界介质中晶体与溶剂的相互作用的预测:通过分子建模控制高过饱和度晶体习性的一种可能方法
这项工作的目的是有助于更好地控制在超临界介质中发生的结晶过程,尤其是在超临界抗溶剂(SAS)过程中。由于使用了分子建模软件GenMol,它的目的还在于改善对晶体习性的预测。工作的第一部分致力于在真空中对两种主要形式的硫代噻唑的晶体建模考虑到哈特曼的依恋能量形式主义。第二部分在整个吸附模拟过程中考虑了溶剂与晶体之间的相互作用,以研究生长环境对晶体习性的影响。最后,将建模预测结果与通过乙腈,丙酮,四氢呋喃和乙酸溶液的SAS工艺重结晶后观察到的噻吩噻唑晶体的生长进行了比较。这些比较表明,考虑到生长环境,对晶体习性的良好预测。二氧化碳(SAS方法的抗溶剂)或乙腈都不会导致在真空中改变等轴测图两种形式的预期习惯。丙酮和四氢呋喃优先吸附在某些已识别的面上,并导致平板,叶状或板状晶体。乙酸吸附在每个表面上,阻碍了相转变为更稳定的形式,从而导致晶体获得了最不稳定,动力学上最受支持的形式I。实验观察也通过考虑不同的可能结晶途径(尤其是结晶)而得到了合理化。 SAS过程中出现的颗粒附着和液滴干燥机制。这项工作证实了溶剂性质是要更好地控制使用SAS工艺生长的颗粒的特性要考虑的关键因素之一,并提供了一种新的方法来帮助控制它。
更新日期:2020-10-07
中文翻译:

超临界介质中晶体与溶剂的相互作用的预测:通过分子建模控制高过饱和度晶体习性的一种可能方法
这项工作的目的是有助于更好地控制在超临界介质中发生的结晶过程,尤其是在超临界抗溶剂(SAS)过程中。由于使用了分子建模软件GenMol,它的目的还在于改善对晶体习性的预测。工作的第一部分致力于在真空中对两种主要形式的硫代噻唑的晶体建模考虑到哈特曼的依恋能量形式主义。第二部分在整个吸附模拟过程中考虑了溶剂与晶体之间的相互作用,以研究生长环境对晶体习性的影响。最后,将建模预测结果与通过乙腈,丙酮,四氢呋喃和乙酸溶液的SAS工艺重结晶后观察到的噻吩噻唑晶体的生长进行了比较。这些比较表明,考虑到生长环境,对晶体习性的良好预测。二氧化碳(SAS方法的抗溶剂)或乙腈都不会导致在真空中改变等轴测图两种形式的预期习惯。丙酮和四氢呋喃优先吸附在某些已识别的面上,并导致平板,叶状或板状晶体。乙酸吸附在每个表面上,阻碍了相转变为更稳定的形式,从而导致晶体获得了最不稳定,动力学上最受支持的形式I。实验观察也通过考虑不同的可能结晶途径(尤其是结晶)而得到了合理化。 SAS过程中出现的颗粒附着和液滴干燥机制。这项工作证实了溶剂性质是要更好地控制使用SAS工艺生长的颗粒的特性要考虑的关键因素之一,并提供了一种新的方法来帮助控制它。


























 京公网安备 11010802027423号
京公网安备 11010802027423号