Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 3.2 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.bbapap.2020.140535 Subhrajyoti Dolai 1 , Sreelakshmi Cherakara 1 , Kanchan Garai 1
|
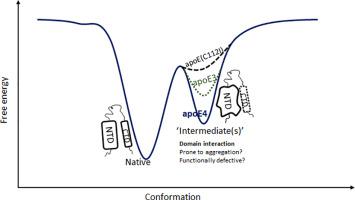
ApoE4(C112R) is the strongest risk factor for Alzheimer's disease, while apoE3(C112) is considered normal. The C112R substitution is believed to alter the interactions between the N-terminal (NTD) and the C-terminal domain (CTD) leading to major functional differences. Here we investigate how the molecular property of the residue at position 112 affects domain interaction using an array of C112X substitutions with arginine, alanine, threonine, valine, leucine and isoleucine as ‘X’. We attempt to determine the free energy of domain interaction (∆GINT) from stabilities of the NTD (∆GNTD) and CTD (∆GCTD) in the full-length apoE, and the stabilities of fragments of the NTD (∆GNTF) and CTD (∆GCTF), using the relationship, ∆GINT = ∆GNTD + ∆GCTD - ∆GNTF - ∆GCTF. We find that although ∆GNTD is strongly dependent on the C112X substitutions, ∆GNTD - ∆GNTF is small. Furthermore, ∆GCTD remains nearly the same as ∆GCTF. Therefore, ∆GINT is estimated to be small and similar for the apoE isoforms. However, stability of domain interaction monitored by urea dependent changes in interdomain Forster Resonance Energy Transfer (FRET) is found to be strongly dependent on C112X substitutions. ApoE4 exhibits the highest mid-point of denaturation of interdomain FRET. To resolve the apparently contradictory observations, we hypothesize that higher interdomain FRET in apoE4 in urea may involve ‘intermediate’ states. Enhanced fluorescence of bis-ANS and susceptibility to proteolytic cleavage support that apoE4, specifically, the NTD of apoE4 harbor ‘intermediates’ in both native and mildly denaturing conditions. The intermediates could hold key to the pathological functions of apoE4.
中文翻译:

载脂蛋白E4展示具有域相互作用的中间体。
ApoE4(C112R)是阿尔茨海默氏病的最强危险因素,而apoE3(C112)被认为是正常的。据信C112R取代改变了N末端(NTD)和C末端结构域(CTD)之间的相互作用,从而导致主要的功能差异。在这里,我们研究了如何使用一系列以精氨酸,丙氨酸,苏氨酸,缬氨酸,亮氨酸和异亮氨酸为“ X”的C112X替代物,对位置112处残基的分子特性如何影响域相互作用。我们尝试根据全长apoE中NTD(∆G NTD)和CTD(∆G CTD)的稳定性以及NTD片段的稳定性(∆G )确定域相互作用的自由能(∆G INT)NTF)和CTD(∆G CTF),使用关系∆G INT = ∆G NTD + ∆G CTD -∆G NTF -∆G CTF。我们发现,尽管∆G NTD强烈依赖于C112X替代,但是∆G NTD -∆G NTF很小。此外,∆G CTD几乎与∆G CTF相同。因此,∆G INT据估计,apoE亚型很小且相似。但是,发现域间福斯特共振能量转移(FRET)中依赖尿素的变化监测的域相互作用的稳定性强烈依赖于C112X取代。ApoE4表现出域间FRET变性的最高中点。为了解决似乎矛盾的观察,我们假设尿素apoE4中较高的域间FRET可能涉及“中间”状态。增强的bis-ANS荧光和对蛋白水解切割的敏感性支持apoE4,特别是apoE4的NTD在天然和轻度变性条件下均具有“中间体”的功能。中间体可能是apoE4病理功能的关键。


























 京公网安备 11010802027423号
京公网安备 11010802027423号