当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
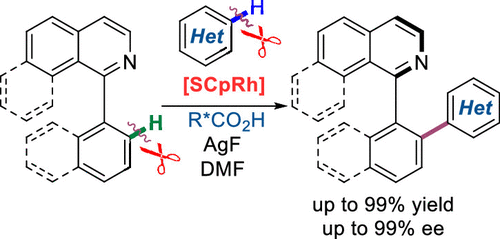
Rhodium-Catalyzed Atroposelective Oxidative C-H/C-H Cross Coupling Reaction of 1-Aryl Isoquinoline Derivatives with Electron-Rich Heteroarenes
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-08-31 , DOI: 10.1021/jacs.0c08205 Qiang Wang 1 , Wen-Wen Zhang 2 , Hao Song 1 , Jian Wang 1 , Chao Zheng 1 , Qing Gu 1 , Shu-Li You 1, 2
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-08-31 , DOI: 10.1021/jacs.0c08205 Qiang Wang 1 , Wen-Wen Zhang 2 , Hao Song 1 , Jian Wang 1 , Chao Zheng 1 , Qing Gu 1 , Shu-Li You 1, 2
Affiliation
|
Rhodium(III)-catalyzed enantioselective oxidative C-H/C-H cross-coupling reaction between two arenes is disclosed. With the combination of a chiral CpRh(III) complex and a chiral carboxylic acid additive, the direct coupling reactions between 1-aryl isoquinoline derivatives and electron-rich heteroarenes such as thiophenes, furans, benzothiophenes, benzofurans are realized via a double C-H functionalization process. A series of axially chiral compounds are obtained in excellent yields and enantioselectivities (up to 99% yield and 99% ee). Mechanistic studies suggest that both C-H bond cleavages may not be the turnover-limiting step.
中文翻译:

铑催化的 1-芳基异喹啉衍生物与富电子杂芳烃的阻转选择性氧化 CH/CH 交叉偶联反应
公开了铑(III)-催化的两种芳烃之间的对映选择性氧化CH/CH交叉偶联反应。通过手性 CpRh(III) 配合物和手性羧酸添加剂的结合,1-芳基异喹啉衍生物与富电子杂芳烃如噻吩、呋喃、苯并噻吩、苯并呋喃之间的直接偶联反应是通过双 CH 官能化过程实现的. 以优异的产率和对映选择性(高达 99% 的产率和 99% ee)获得了一系列轴向手性化合物。机理研究表明,两个 CH 键裂解可能不是周转限制步骤。
更新日期:2020-08-31
中文翻译:

铑催化的 1-芳基异喹啉衍生物与富电子杂芳烃的阻转选择性氧化 CH/CH 交叉偶联反应
公开了铑(III)-催化的两种芳烃之间的对映选择性氧化CH/CH交叉偶联反应。通过手性 CpRh(III) 配合物和手性羧酸添加剂的结合,1-芳基异喹啉衍生物与富电子杂芳烃如噻吩、呋喃、苯并噻吩、苯并呋喃之间的直接偶联反应是通过双 CH 官能化过程实现的. 以优异的产率和对映选择性(高达 99% 的产率和 99% ee)获得了一系列轴向手性化合物。机理研究表明,两个 CH 键裂解可能不是周转限制步骤。



























 京公网安备 11010802027423号
京公网安备 11010802027423号