当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
C5 conserved region of hydrophilic C‐terminal part of Saccharomyces cerevisiae Nha1 antiporter determines its requirement of Erv14 COPII cargo receptor for plasma‐membrane targeting
Molecular Microbiology ( IF 3.6 ) Pub Date : 2020-08-31 , DOI: 10.1111/mmi.14595 Klara Papouskova 1 , Michaela Moravcova 1 , Gal Masrati 2 , Nir Ben-Tal 2 , Hana Sychrova 1 , Olga Zimmermannova 1
Molecular Microbiology ( IF 3.6 ) Pub Date : 2020-08-31 , DOI: 10.1111/mmi.14595 Klara Papouskova 1 , Michaela Moravcova 1 , Gal Masrati 2 , Nir Ben-Tal 2 , Hana Sychrova 1 , Olga Zimmermannova 1
Affiliation
|
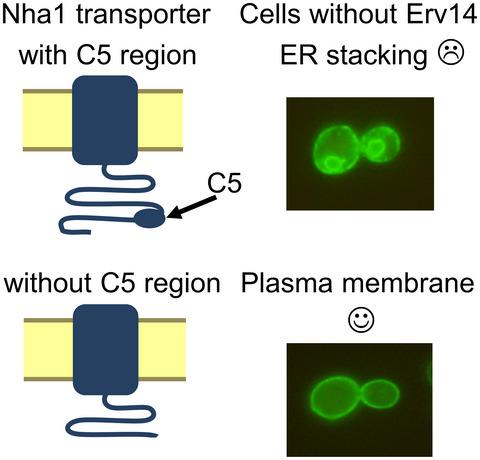
Erv14, a conserved cargo receptor of COPII vesicles, helps the proper trafficking of many but not all transporters to the yeast plasma membrane, for example, three out of five alkali‐metal‐cation transporters in Saccharomyces cerevisiae. Among them, the Nha1 cation/proton antiporter, which participates in cell cation and pH homeostasis, is a large membrane protein (985 aa) possessing a long hydrophilic C‐terminus (552 aa) containing six conserved regions (C1–C6) with unknown function. A short Nha1 version, lacking almost the entire C‐terminus, still binds to Erv14 but does not need it to be targeted to the plasma membrane. Comparing the localization and function of ScNha1 variants shortened at its C‐terminus in cells with or without Erv14 reveals that only ScNha1 versions possessing the complete C5 region are dependent on Erv14. In addition, our broad evolutionary conservation analysis of fungal Na+/H+ antiporters identified new conserved regions in their C‐termini, and our experiments newly show C5 and other, so far unknown, regions of the C‐terminus, to be involved in the functionality and substrate specificity of ScNha1. Taken together, our results reveal that also relatively small hydrophilic parts of some yeast membrane proteins underlie their need to interact with the Erv14 cargo receptor.
中文翻译:

酿酒酵母 Nha1 反向转运蛋白亲水 C 端部分的 C5 保守区决定了其对质膜靶向的 Erv14 COPII 货物受体的需求
Erv14 是 COPII 囊泡的保守货物受体,有助于将许多但不是所有转运蛋白正确运输到酵母质膜,例如,酿酒酵母中五分之三的碱金属阳离子转运蛋白。其中,参与细胞阳离子和 pH 稳态的 Nha1 阳离子/质子反向转运蛋白是一种大膜蛋白 (985 aa),具有长亲水 C 端 (552 aa),其中包含六个未知的保守区域 (C1-C6)。功能。一个短的 Nha1 版本,几乎没有整个 C 端,仍然与 Erv14 结合,但不需要它靶向质膜。比较在有或没有 Erv14 的细胞中 C 端缩短的Sc Nha1 变体的定位和功能表明,只有Sc拥有完整 C5 区域的 Nha1 版本依赖于 Erv14。此外,我们对真菌 Na + /H +逆向转运蛋白的广泛进化保守性分析在其 C 端发现了新的保守区域,我们的实验新显示 C5 和 C 端的其他迄今为止未知的区域参与Sc Nha1的功能和底物特异性。总之,我们的结果表明,一些酵母膜蛋白的亲水部分也相对较小,这也是它们需要与 Erv14 货物受体相互作用的基础。
更新日期:2020-08-31
中文翻译:

酿酒酵母 Nha1 反向转运蛋白亲水 C 端部分的 C5 保守区决定了其对质膜靶向的 Erv14 COPII 货物受体的需求
Erv14 是 COPII 囊泡的保守货物受体,有助于将许多但不是所有转运蛋白正确运输到酵母质膜,例如,酿酒酵母中五分之三的碱金属阳离子转运蛋白。其中,参与细胞阳离子和 pH 稳态的 Nha1 阳离子/质子反向转运蛋白是一种大膜蛋白 (985 aa),具有长亲水 C 端 (552 aa),其中包含六个未知的保守区域 (C1-C6)。功能。一个短的 Nha1 版本,几乎没有整个 C 端,仍然与 Erv14 结合,但不需要它靶向质膜。比较在有或没有 Erv14 的细胞中 C 端缩短的Sc Nha1 变体的定位和功能表明,只有Sc拥有完整 C5 区域的 Nha1 版本依赖于 Erv14。此外,我们对真菌 Na + /H +逆向转运蛋白的广泛进化保守性分析在其 C 端发现了新的保守区域,我们的实验新显示 C5 和 C 端的其他迄今为止未知的区域参与Sc Nha1的功能和底物特异性。总之,我们的结果表明,一些酵母膜蛋白的亲水部分也相对较小,这也是它们需要与 Erv14 货物受体相互作用的基础。



























 京公网安备 11010802027423号
京公网安备 11010802027423号