Journal of CO2 Utilization ( IF 7.7 ) Pub Date : 2020-08-31 , DOI: 10.1016/j.jcou.2020.101292 Redouane Melouki , Amina Ouadah , Philip L. Llewellyn
|
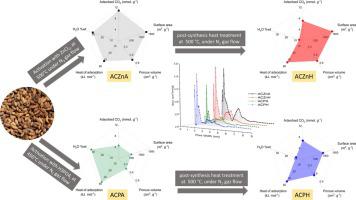
In the present framework, we have studied the relationship between the activated carbon surface characteristics and the CO2 adsorption behavior. The two activated carbon series were synthesized from olive waste as a precursor employing ZnCl2 and H3PO4 as chemical activation agents; their yield of production reaches 34.2 % and 47.3 % respectively. Adsorption isotherms were carried out at 20 °C, 30 °C, 40 °C and 50 °C and pressured up to 1.48 MPa via manometric measurement. Activation agent and post-synthesis heat treatment effects have been investigated. The CO2 maximum amount uptake was equal to 10.96 mmol. g−1 and was achieved on the activated sample with ZnCl2 having a specific surface area about 1399 m2. g-1. Although, the synthesized activated carbon through the H3PO4 activation agent has less surface area, their adsorption per unit area is in the same range as the activated sample with ZnCl2.
Four adsorption models were applied to modulate the CO2 adsorption behavior on the activated carbon surface (Langmuir, dual Langmuir, Sips and Toth isotherms). Models considering surface heterogeneity lead to a good correlation with adsorption data. Furthermore, the adsorption heats are measured and compared to the isosteric heats estimated from the isotherm model equation as well as the calculated ones using the Clausius − Clapeyron equation. The Langmuir model provides the nearest values to the measured adsorption heat, these value are about .
中文翻译:

橄榄渣合成活性炭对CO 2的吸附行为研究
在目前的框架下,我们研究了活性炭表面特性与CO 2吸附行为之间的关系。以ZnCl 2和H 3 PO 4为化学活化剂,以橄榄渣为前驱体合成了两种活性炭。它们的产量分别达到34.2%和47.3%。吸附等温线在20°C,30°C,40°C和50°C下进行,并通过测压法加压至1.48 MPa。已经研究了活化剂和合成后的热处理效果。CO 2的最大吸收量等于10.96 mmol。g -1并用ZnCl 2活化样品具有约1399m 2的比表面积。g -1。尽管通过H 3 PO 4活化剂合成的活性炭具有较小的表面积,但是它们的每单位面积的吸附在与具有ZnCl 2的活性样品相同的范围内。
应用了四种吸附模型来调节活性炭表面上的CO 2吸附行为(Langmuir,双重Langmuir,Sips和Toth等温线)。考虑表面异质性的模型导致与吸附数据的良好相关性。此外,测量吸附热,并将其与根据等温模型方程估算的等规热量以及使用克劳修斯-克拉佩隆方程计算的等热量进行比较。Langmuir模型提供了最接近所测吸附热的值,这些值约为。


























 京公网安备 11010802027423号
京公网安备 11010802027423号