当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Directing-Group-Assisted C(sp2)-H Arylsulfonylation from Sulfur Dioxide.
Organic Letters ( IF 5.2 ) Pub Date : 2020-08-28 , DOI: 10.1021/acs.orglett.0c02400 Tonghao Zhu 1 , Jie Wu 1, 2
Organic Letters ( IF 5.2 ) Pub Date : 2020-08-28 , DOI: 10.1021/acs.orglett.0c02400 Tonghao Zhu 1 , Jie Wu 1, 2
Affiliation
|
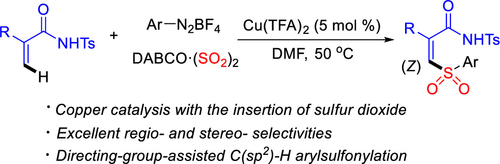
A straightforward and stereoselective preparation of (Z)-β-alkenyl sulfones through a reaction of N-tosyl acrylamides, 1,4-diazabicyclo[2.2.2]octane-sulfur dioxide, and aryldiazonium tetrafluoroborates under copper catalysis is accomplished. The direct C(sp2)–H arylsulfonylation of acrylamides using sulfur dioxide as the sulfonyl source in the presence of copper trifluoroacetate proceeds smoothly, giving rise to (Z)-β-alkenyl sulfones in good yields. During the reaction process, excellent regio- and stereoselectivities are observed.
中文翻译:

指导小组协助二氧化硫的C(sp2)-H芳基磺酰化。
通过在铜催化下的N-甲苯磺酰基丙烯酰胺,1,4-二氮杂双环[2.2.2]辛烷-二氧化硫和芳基重氮四氟硼酸酯的反应,完成了(Z)-β-烯基砜的直接和立体选择性制备。在三氟乙酸铜存在下,使用二氧化硫作为磺酰基源,丙烯酰胺的直接C(sp 2)-H芳基磺酰化反应顺利进行,从而以高收率产生了(Z)-β-烯基砜。在反应过程中,观察到优异的区域选择性和立体选择性。
更新日期:2020-09-20
中文翻译:

指导小组协助二氧化硫的C(sp2)-H芳基磺酰化。
通过在铜催化下的N-甲苯磺酰基丙烯酰胺,1,4-二氮杂双环[2.2.2]辛烷-二氧化硫和芳基重氮四氟硼酸酯的反应,完成了(Z)-β-烯基砜的直接和立体选择性制备。在三氟乙酸铜存在下,使用二氧化硫作为磺酰基源,丙烯酰胺的直接C(sp 2)-H芳基磺酰化反应顺利进行,从而以高收率产生了(Z)-β-烯基砜。在反应过程中,观察到优异的区域选择性和立体选择性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号