当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Loxoprofen Solubility in Supercritical Carbon Dioxide: Experimental and Modeling Approaches
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-08-28 , DOI: 10.1021/acs.jced.0c00470 Samyar Zabihi 1 , Seyyed Hamid Esmaeili-Faraj 2 , Fatemeh Borousan 3, 4, 5 , Ali Zeinolabedini Hezave 3, 4 , Saeed Shirazian 6, 7
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-08-28 , DOI: 10.1021/acs.jced.0c00470 Samyar Zabihi 1 , Seyyed Hamid Esmaeili-Faraj 2 , Fatemeh Borousan 3, 4, 5 , Ali Zeinolabedini Hezave 3, 4 , Saeed Shirazian 6, 7
Affiliation
|
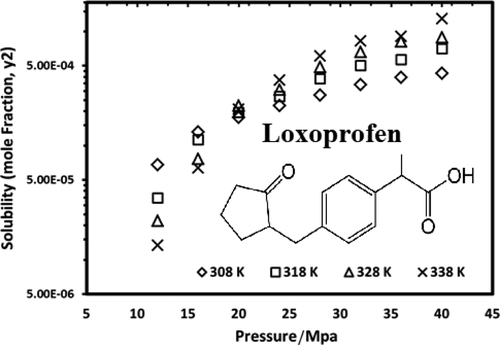
The solubility of loxoprofen as a nonsteroidal anti-inflammatory drug (NSAID) is measured at various temperatures (308, 318, 328, and 338 K) and pressures (12, 16, 20, 24, 28, 32, 36, and 40 MPa) in supercritical carbon dioxide (SC-CO2). The solubility data were measured using a gravimetric-based approach and revealed the solubility range of 1.35 × 10–5 to 1.28 × 10–3 based on the mole fraction of loxoprofen. The results revealed that solubility can be significantly enhanced from 1.04 × 10–5 to 1.28 × 10–3 (mole fraction basis) for the isotherm at 338 K because of the effect of temperature which can boost the pressure effect on solubility enhancement, at pressures greater than crossover (around 20 MPa for the case of loxoprofen). Moreover, the experimental data points were modeled using five different density-based correlations including Chrastil, Garlapati and Madras, Mendez-Santiago and Teja (MST), Bartle et al., and Kumar and Johnston (K–J) models because measuring the solubility of loxoprofen in entire required ranges of pressure and temperature is impossible or expensive similar to the other pharmaceuticals. The results of modeling revealed that one can correlate the loxoprofen solubility data with an accuracy of about 9.2% (Mendez-Santiago–Teja), 10.7% (Bartle et al.), 7.1% (Kumar and Johnstone), 12.7% (Chrastil), and 12.7% (Garlapati and Madras) based on average absolute relative deviation percent (AARD %).
中文翻译:

洛索洛芬在超临界二氧化碳中的溶解度:实验和建模方法
在各种温度(308、318、328和338 K)和压力(12、16、20、24、28、32、36和40 MPa)下测量洛索洛芬作为非甾体类抗炎药(NSAID)的溶解度)在超临界二氧化碳(SC-CO 2)中。溶解度数据使用基于重量法的方法进行测量,基于洛索洛芬的摩尔分数,得出的溶解度范围为1.35×10 –5至1.28×10 –3。结果表明,溶解度可以从1.04×10 –5显着提高到1.28×10 –3(摩尔分数基础)在338 K时是等温线,这是因为温度的影响可以提高压力对溶解度的提高,而压力大于交叉点(洛索洛芬的情况下约为20 MPa)。此外,使用五个不同的基于密度的相关性对实验数据点进行建模,包括Chrastil,Garlapati和Madras,Mendez-Santiago和Teja(MST),Bartle等人以及Kumar和Johnston(K–J)模型,因为可测量溶解度与其他药物类似,在所有所需的压力和温度范围内使用洛索洛芬是不可能的,或者是昂贵的。建模结果表明,可以将洛索洛芬溶解度数据与以下数据相关联:准确度约为9.2%(Mendez-Santiago-Teja),10.7%(Bartle等),7.1%(Kumar和Johnstone),12.7%(Chrastil)和12。
更新日期:2020-09-10
中文翻译:

洛索洛芬在超临界二氧化碳中的溶解度:实验和建模方法
在各种温度(308、318、328和338 K)和压力(12、16、20、24、28、32、36和40 MPa)下测量洛索洛芬作为非甾体类抗炎药(NSAID)的溶解度)在超临界二氧化碳(SC-CO 2)中。溶解度数据使用基于重量法的方法进行测量,基于洛索洛芬的摩尔分数,得出的溶解度范围为1.35×10 –5至1.28×10 –3。结果表明,溶解度可以从1.04×10 –5显着提高到1.28×10 –3(摩尔分数基础)在338 K时是等温线,这是因为温度的影响可以提高压力对溶解度的提高,而压力大于交叉点(洛索洛芬的情况下约为20 MPa)。此外,使用五个不同的基于密度的相关性对实验数据点进行建模,包括Chrastil,Garlapati和Madras,Mendez-Santiago和Teja(MST),Bartle等人以及Kumar和Johnston(K–J)模型,因为可测量溶解度与其他药物类似,在所有所需的压力和温度范围内使用洛索洛芬是不可能的,或者是昂贵的。建模结果表明,可以将洛索洛芬溶解度数据与以下数据相关联:准确度约为9.2%(Mendez-Santiago-Teja),10.7%(Bartle等),7.1%(Kumar和Johnstone),12.7%(Chrastil)和12。


























 京公网安备 11010802027423号
京公网安备 11010802027423号