当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Activity Mapping the Acyl Carrier Protein: Elongating Ketosynthase Interaction in Fatty Acid Biosynthesis.
Biochemistry ( IF 2.9 ) Pub Date : 2020-08-28 , DOI: 10.1021/acs.biochem.0c00605 Jeffrey T Mindrebo 1, 2 , Laetitia E Misson 1 , Caitlin Johnson 1 , Joseph P Noel 2 , Michael D Burkart 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-08-28 , DOI: 10.1021/acs.biochem.0c00605 Jeffrey T Mindrebo 1, 2 , Laetitia E Misson 1 , Caitlin Johnson 1 , Joseph P Noel 2 , Michael D Burkart 1
Affiliation
|
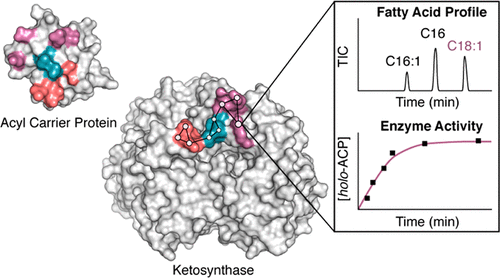
Elongating ketosynthases (KSs) catalyze carbon–carbon bond-forming reactions during the committed step for each round of chain extension in both fatty acid synthases (FASs) and polyketide synthases (PKSs). A small α-helical acyl carrier protein (ACP) shuttles fatty acyl intermediates between enzyme active sites. To accomplish this task, the ACP relies on a series of dynamic interactions with multiple partner enzymes of FAS and associated FAS-dependent pathways. Recent structures of the Escherichia coli FAS ACP, AcpP, in covalent complexes with its two cognate elongating KSs, FabF and FabB, provide high-resolution details of these interfaces, but a systematic analysis of specific interfacial interactions responsible for stabilizing these complexes has not yet been undertaken. Here, we use site-directed mutagenesis with both in vitro and in vivo activity analyses to quantitatively evaluate these contacting surfaces between AcpP and FabF. We delineate the FabF interface into three interacting regions and demonstrate the effects of point mutants, double mutants, and region deletion variants. Results from these analyses reveal a robust and modular FabF interface capable of tolerating seemingly critical interface mutations with only the deletion of an entire region significantly compromising activity. Structure and sequence analyses of FabF orthologs from related type II FAS pathways indicate significant conservation of type II FAS KS interface residues and, overall, support its delineation into interaction regions. These findings strengthen our mechanistic understanding of molecular recognition events between ACPs and FAS enzymes and provide a blueprint for engineering ACP-dependent biosynthetic pathways.
中文翻译:

酰基载体蛋白的活性作图:延长脂肪酸生物合成中的酮合酶相互作用。
在脂肪酸合酶 (FAS) 和聚酮化合物合酶 (PKS) 的每一轮链延伸的关键步骤中,延长酮合酶 (KSs) 催化碳-碳键形成反应。一个小的 α-螺旋酰基载体蛋白 (ACP) 在酶活性位点之间穿梭脂肪酰基中间体。为了完成这项任务,ACP 依赖于与 FAS 和相关 FAS 依赖途径的多种伙伴酶的一系列动态相互作用。大肠杆菌的最新结构FAS ACP、AcpP 与其两个同源伸长的 KS FabF 和 FabB 形成共价复合物,提供了这些界面的高分辨率细节,但尚未对负责稳定这些复合物的特定界面相互作用进行系统分析。在这里,我们在体外和体内使用定点诱变活性分析以定量评估 AcpP 和 FabF 之间的这些接触表面。我们将 FabF 界面划分为三个相互作用的区域,并展示了点突变体、双突变体和区域缺失变体的影响。这些分析的结果揭示了一个强大且模块化的 FabF 界面,能够容忍看似关键的界面突变,而仅删除整个区域就会显着影响活性。来自相关 II 型 FAS 通路的 FabF 直向同源物的结构和序列分析表明 II 型 FAS KS 界面残基的显着保守性,并且总体上支持将其划分为相互作用区域。
更新日期:2020-09-29
中文翻译:

酰基载体蛋白的活性作图:延长脂肪酸生物合成中的酮合酶相互作用。
在脂肪酸合酶 (FAS) 和聚酮化合物合酶 (PKS) 的每一轮链延伸的关键步骤中,延长酮合酶 (KSs) 催化碳-碳键形成反应。一个小的 α-螺旋酰基载体蛋白 (ACP) 在酶活性位点之间穿梭脂肪酰基中间体。为了完成这项任务,ACP 依赖于与 FAS 和相关 FAS 依赖途径的多种伙伴酶的一系列动态相互作用。大肠杆菌的最新结构FAS ACP、AcpP 与其两个同源伸长的 KS FabF 和 FabB 形成共价复合物,提供了这些界面的高分辨率细节,但尚未对负责稳定这些复合物的特定界面相互作用进行系统分析。在这里,我们在体外和体内使用定点诱变活性分析以定量评估 AcpP 和 FabF 之间的这些接触表面。我们将 FabF 界面划分为三个相互作用的区域,并展示了点突变体、双突变体和区域缺失变体的影响。这些分析的结果揭示了一个强大且模块化的 FabF 界面,能够容忍看似关键的界面突变,而仅删除整个区域就会显着影响活性。来自相关 II 型 FAS 通路的 FabF 直向同源物的结构和序列分析表明 II 型 FAS KS 界面残基的显着保守性,并且总体上支持将其划分为相互作用区域。



























 京公网安备 11010802027423号
京公网安备 11010802027423号