当前位置:
X-MOL 学术
›
ACS Macro Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chain-Growth Polymerization of Benzotriazole Using Suzuki–Miyaura Cross-Coupling and Dialkylbiarylphosphine Palladium Catalysts
ACS Macro Letters ( IF 5.8 ) Pub Date : 2020-08-28 , DOI: 10.1021/acsmacrolett.0c00580 Michael V Bautista 1 , Anthony J Varni 1 , Josué Ayuso-Carrillo 1 , Chia-Hua Tsai 1 , Kevin J T Noonan 1
ACS Macro Letters ( IF 5.8 ) Pub Date : 2020-08-28 , DOI: 10.1021/acsmacrolett.0c00580 Michael V Bautista 1 , Anthony J Varni 1 , Josué Ayuso-Carrillo 1 , Chia-Hua Tsai 1 , Kevin J T Noonan 1
Affiliation
|
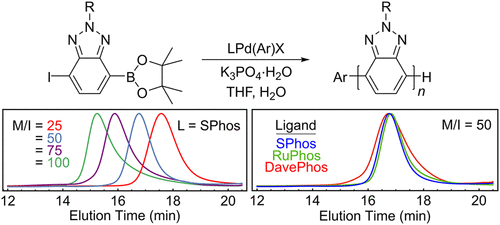
Electron-deficient (n-type) conjugated materials are commonly prepared via step-growth methods with limited control over the molecular weight and molecular weight distribution of the resulting polymers. In this communication, we demonstrate that Pd-dialkylbiarylphosphine catalysts enable the chain-growth polymerization of benzo[1,2,3]triazole using Suzuki-Miyaura coupling with molecular weight control and modest molecular weight distributions (Đ ∼ 1.2–1.6). The importance of a free ligand in the reaction mixture during polymerization was established by analysis of polymer samples using GPC and MALDI-TOF mass spectrometry. A block copolymer with poly(3-hexylthiophene) was also synthesized by sequential monomer addition. The success of these commercially available catalysts for polymerization of benzotriazole highlights their potential for chain-growth reactions with other bicyclic arenes in the future.
中文翻译:

使用 Suzuki-Miyaura 交叉偶联和二烷基联芳基膦钯催化剂的苯并三唑链增长聚合
缺电子(n 型)共轭材料通常通过逐步增长的方法制备,对所得聚合物的分子量和分子量分布的控制有限。在本次交流中,我们证明了 Pd-二烷基联芳基膦催化剂能够使用 Suzuki-Miyaura 偶联实现苯并[1,2,3]三唑的链增长聚合,同时具有分子量控制和适度的分子量分布 ( Đ~ 1.2-1.6)。通过使用 GPC 和 MALDI-TOF 质谱法分析聚合物样品,确定了聚合过程中反应混合物中游离配体的重要性。还通过顺序单体添加合成了具有聚 (3-己基噻吩) 的嵌段共聚物。这些用于苯并三唑聚合的市售催化剂的成功凸显了它们在未来与其他双环芳烃进行链增长反应的潜力。
更新日期:2020-09-15
中文翻译:

使用 Suzuki-Miyaura 交叉偶联和二烷基联芳基膦钯催化剂的苯并三唑链增长聚合
缺电子(n 型)共轭材料通常通过逐步增长的方法制备,对所得聚合物的分子量和分子量分布的控制有限。在本次交流中,我们证明了 Pd-二烷基联芳基膦催化剂能够使用 Suzuki-Miyaura 偶联实现苯并[1,2,3]三唑的链增长聚合,同时具有分子量控制和适度的分子量分布 ( Đ~ 1.2-1.6)。通过使用 GPC 和 MALDI-TOF 质谱法分析聚合物样品,确定了聚合过程中反应混合物中游离配体的重要性。还通过顺序单体添加合成了具有聚 (3-己基噻吩) 的嵌段共聚物。这些用于苯并三唑聚合的市售催化剂的成功凸显了它们在未来与其他双环芳烃进行链增长反应的潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号