当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
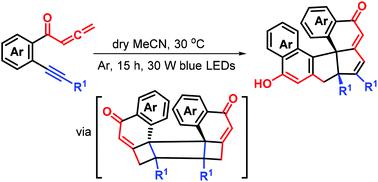
Photoinduced double [2 + 2] cycloaddition relay of yne–allenones for highly diastereoselective synthesis of hexacyclic 1-naphthols
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-08-28 , DOI: 10.1039/d0qo00917b Shan-Shan Zhu 1, 2, 3, 4, 5 , Jiang-Nan Zhou 1, 2, 3, 4, 5 , Quan-Long Wu 1, 2, 3, 4, 5 , Wen-Juan Hao 1, 2, 3, 4, 5 , Shu-Jiang Tu 1, 2, 3, 4, 5 , Bo Jiang 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-08-28 , DOI: 10.1039/d0qo00917b Shan-Shan Zhu 1, 2, 3, 4, 5 , Jiang-Nan Zhou 1, 2, 3, 4, 5 , Quan-Long Wu 1, 2, 3, 4, 5 , Wen-Juan Hao 1, 2, 3, 4, 5 , Shu-Jiang Tu 1, 2, 3, 4, 5 , Bo Jiang 1, 2, 3, 4, 5
Affiliation
|
A new photoinduced photocatalyst-free energy-transfer strategy for double [2 + 2] cycloaddition relay of yne–allenones is reported for the first time and used to produce a series of hitherto unreported hexacyclic 1-naphthols with good yields and complete diastereoselectivity. The reaction pathway generates a class of complex three-dimensional structures with two all-carbon quaternary centers evolved from the planar conjugated system through cleavage and recombination of the C–C triple bond of yne–allenones, enabling the direct formation of four new rings in one step.
中文翻译:

炔烃-炔烃的光诱导双[2 + 2]环加成中继,用于非对映选择性合成六环1-萘酚
首次报道了一种新的无光催化的无光催化剂,用于炔烃-双烯丙基双[2 + 2]环加成中继,可用于生产一系列迄今未报道的六环1-萘,具有良好的产率和完全的非对映选择性。反应路径生成一类复杂的三维结构,具有两个全碳四元中心,这些平面是从平面共轭体系通过炔-烯酮的CC三键的裂解和重组而形成的,从而可以直接在环中形成四个新的环一步。
更新日期:2020-09-30
中文翻译:

炔烃-炔烃的光诱导双[2 + 2]环加成中继,用于非对映选择性合成六环1-萘酚
首次报道了一种新的无光催化的无光催化剂,用于炔烃-双烯丙基双[2 + 2]环加成中继,可用于生产一系列迄今未报道的六环1-萘,具有良好的产率和完全的非对映选择性。反应路径生成一类复杂的三维结构,具有两个全碳四元中心,这些平面是从平面共轭体系通过炔-烯酮的CC三键的裂解和重组而形成的,从而可以直接在环中形成四个新的环一步。


























 京公网安备 11010802027423号
京公网安备 11010802027423号