当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A benzoxazole derivative as an inhibitor of anaerobic choline metabolism by human gut microbiota
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2020-08-28 , DOI: 10.1039/d0md00218f Moustafa T Gabr 1 , David Machalz 2 , Szymon Pach 2 , Gerhard Wolber 2
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2020-08-28 , DOI: 10.1039/d0md00218f Moustafa T Gabr 1 , David Machalz 2 , Szymon Pach 2 , Gerhard Wolber 2
Affiliation
|
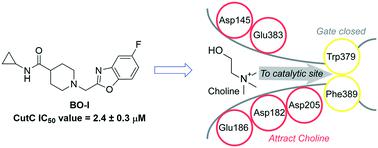
Metabolic pathways mediated by human gut bacteria have emerged as potential therapeutic targets because of their association with the pathophysiology of various human diseases. The anaerobic transformation of choline into trimethylamine (TMA) by gut microbiota is directly linked to type 2 diabetes, fatty liver disease, and cardiovascular diseases. Structural analogs of choline have been developed as competitive inhibitors of choline TMA-lyase (CutC), a key enzyme for the conversion of choline to TMA. However, weak to moderate CutC inhibitory profiles of the choline analogs limit their further advancement into clinical translation. In this study, we introduce a glycomimetic-based approach for the identification of CutC inhibitors with intestinal metabolic stability. Our workflow started with screening of a small library of glycomimetics for metabolic stability in the presence of human intestinal S9 fraction. Further screening using an in vitro CutC inhibitory assay identified a benzoxazole ligand (BO-I) as a CutC inhibitor with an IC50 value of 2.4 ± 0.3 μM. Kinetic analysis revealed that BO-I functions as a non-competitive inhibitor of CutC. Interestingly, BO-I reduced the production of TMA in whole cell assays of multiple bacterial strains as well as in complex biological environments. Therefore, structural optimization of BO-I holds promise for the development of efficient gut microbiota-targeted small molecules.
中文翻译:

苯并恶唑衍生物作为人体肠道微生物群厌氧胆碱代谢的抑制剂
由人类肠道细菌介导的代谢途径已成为潜在的治疗靶点,因为它们与各种人类疾病的病理生理学相关。肠道微生物群将胆碱无氧转化为三甲胺 (TMA),与 2 型糖尿病、脂肪肝疾病和心血管疾病直接相关。胆碱的结构类似物已被开发为胆碱 TMA 裂解酶 (CutC) 的竞争性抑制剂,胆碱 TMA 裂解酶是胆碱转化为 TMA 的关键酶。然而,胆碱类似物的弱至中度 CutC 抑制特性限制了它们进一步进入临床转化。在这项研究中,我们引入了一种基于糖模拟物的方法来鉴定具有肠道代谢稳定性的 CutC 抑制剂。我们的工作流程始于筛选一个小型糖模拟物库,以确保在人肠道 S9 组分存在的情况下的代谢稳定性。使用体外CutC 抑制测定进行进一步筛选,确定苯并恶唑配体 (BO-I) 作为 CutC 抑制剂,IC 50值为 2.4 ± 0.3 μM。动力学分析表明 BO-I 作为 CutC 的非竞争性抑制剂发挥作用。有趣的是,BO-I 在多种细菌菌株的全细胞分析以及复杂的生物环境中减少了 TMA 的产生。因此,BO-I 的结构优化有望开发高效的肠道微生物靶向小分子。
更新日期:2020-11-03
中文翻译:

苯并恶唑衍生物作为人体肠道微生物群厌氧胆碱代谢的抑制剂
由人类肠道细菌介导的代谢途径已成为潜在的治疗靶点,因为它们与各种人类疾病的病理生理学相关。肠道微生物群将胆碱无氧转化为三甲胺 (TMA),与 2 型糖尿病、脂肪肝疾病和心血管疾病直接相关。胆碱的结构类似物已被开发为胆碱 TMA 裂解酶 (CutC) 的竞争性抑制剂,胆碱 TMA 裂解酶是胆碱转化为 TMA 的关键酶。然而,胆碱类似物的弱至中度 CutC 抑制特性限制了它们进一步进入临床转化。在这项研究中,我们引入了一种基于糖模拟物的方法来鉴定具有肠道代谢稳定性的 CutC 抑制剂。我们的工作流程始于筛选一个小型糖模拟物库,以确保在人肠道 S9 组分存在的情况下的代谢稳定性。使用体外CutC 抑制测定进行进一步筛选,确定苯并恶唑配体 (BO-I) 作为 CutC 抑制剂,IC 50值为 2.4 ± 0.3 μM。动力学分析表明 BO-I 作为 CutC 的非竞争性抑制剂发挥作用。有趣的是,BO-I 在多种细菌菌株的全细胞分析以及复杂的生物环境中减少了 TMA 的产生。因此,BO-I 的结构优化有望开发高效的肠道微生物靶向小分子。



























 京公网安备 11010802027423号
京公网安备 11010802027423号