当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Microsolvation of Sodium Thiocyanate in Water: Gas Phase Anion Photoelectron Spectroscopy and Theoretical Calculations.
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2020-08-27 , DOI: 10.1021/acs.jpca.0c07071 Shiyan Gong 1, 2 , Peng Wang 1, 2 , Zhiyou Wei 1, 2 , Bin Yang 1, 2 , Xiling Xu 1, 2 , Hongguang Xu 1, 2 , Weijun Zheng 1, 2
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2020-08-27 , DOI: 10.1021/acs.jpca.0c07071 Shiyan Gong 1, 2 , Peng Wang 1, 2 , Zhiyou Wei 1, 2 , Bin Yang 1, 2 , Xiling Xu 1, 2 , Hongguang Xu 1, 2 , Weijun Zheng 1, 2
Affiliation
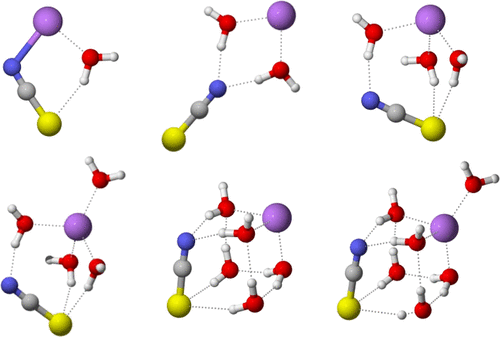
|
We studied NaSCN(H2O)n– clusters in the gas phase using size-selected anion photoelectron spectroscopy. Vertical detachment energies (VDEs) of NaSCN(H2O)n– (n = 0–6) clusters were obtained in the experiment. Structures of NaSCN(H2O)n– (n = 0–6) clusters and their corresponding neutral counterparts were investigated by theoretical calculations. Our studies show that the Na–NCS type of structures rather than the Na–SCN type of structures are dominant in the gas phase for both anionic and neutral NaSCN(H2O)n clusters. For NaSCN(H2O)n– anions, the contact ion pair (CIP) and solvent-separated ion pair (SSIP) type of structures coexist at n = 2 and 3, the SSIP type of structures become dominant at n ≥ 4. For neutral NaSCN(H2O)n, the CIP type of structures are dominant from n = 1 to 6 and the SSIP type of structures start to appear at n = 6. The gas phase structures in this work support the existence of ion clustering in concentrated NaSCN aqueous solutions.
中文翻译:

硫氰酸钠在水中的微溶剂化:气相阴离子光电子能谱和理论计算。
我们研究的NaSCN(H 2 O)ñ -使用大小选择的阴离子光电子能谱法在气相中的簇。在实验中获得了NaSCN(H 2 O)n –(n = 0-6)团簇的垂直脱离能(VDE)。通过理论计算研究了NaSCN(H 2 O)n –(n = 0-6)团簇的结构及其对应的中性对应物。我们的研究表明,对于阴离子和中性NaSCN(H 2 O)n团簇,Na–NCS型结构而非Na–SCN型结构在气相中均占主导地位。对于NaSCN(H 2O)ñ -阴离子,接触离子对(CIP)和溶剂分离的离子对(SSIP)型在结构共存的Ñ = 2和图3,结构的SSIP类型成为主导Ñ ≥4.对于中性的NaSCN(H 2 O)n时,CIP类型的结构从n = 1到6占主导地位,SSIP类型的结构在n = 6时开始出现。这项工作中的气相结构支持浓NaSCN水溶液中离子簇的存在。 。
更新日期:2020-09-24
中文翻译:

硫氰酸钠在水中的微溶剂化:气相阴离子光电子能谱和理论计算。
我们研究的NaSCN(H 2 O)ñ -使用大小选择的阴离子光电子能谱法在气相中的簇。在实验中获得了NaSCN(H 2 O)n –(n = 0-6)团簇的垂直脱离能(VDE)。通过理论计算研究了NaSCN(H 2 O)n –(n = 0-6)团簇的结构及其对应的中性对应物。我们的研究表明,对于阴离子和中性NaSCN(H 2 O)n团簇,Na–NCS型结构而非Na–SCN型结构在气相中均占主导地位。对于NaSCN(H 2O)ñ -阴离子,接触离子对(CIP)和溶剂分离的离子对(SSIP)型在结构共存的Ñ = 2和图3,结构的SSIP类型成为主导Ñ ≥4.对于中性的NaSCN(H 2 O)n时,CIP类型的结构从n = 1到6占主导地位,SSIP类型的结构在n = 6时开始出现。这项工作中的气相结构支持浓NaSCN水溶液中离子簇的存在。 。


























 京公网安备 11010802027423号
京公网安备 11010802027423号