当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Spectroscopic Quantification of Inner- and Outer-Sphere Oxyanion Complexation Kinetics: Ionic Strength and Background Cation Effect on Sulfate Adsorption to Hematite
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2020-08-27 , DOI: 10.1021/acsearthspacechem.0c00149 Michael P. Schmidt 1 , Steven D. Siciliano 1 , Derek Peak 1
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2020-08-27 , DOI: 10.1021/acsearthspacechem.0c00149 Michael P. Schmidt 1 , Steven D. Siciliano 1 , Derek Peak 1
Affiliation
|
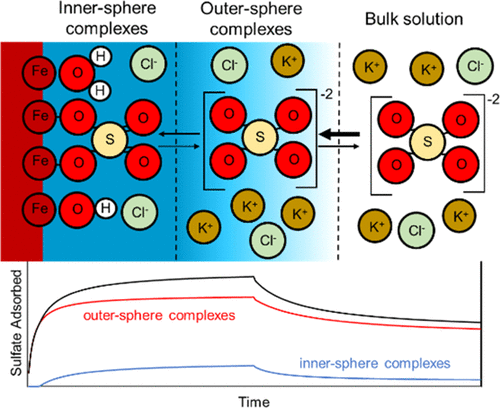
Sulfate adsorbs on Fe-oxide minerals by both inner- and outer-sphere modes; however, the time dependence of coexisting surface species is not clear. Using in situ attenuated total reflectance–Fourier transform infrared spectroscopy, peak fitting, and multivariate curve resolution analyses, we quantify adsorption and desorption kinetics of inner- and outer-sphere sulfate species on hematite at two ionic strengths (I = 0.01 and 0.10 M) and background cations (K+ and Ca2+) at pH 4.5. We experimentally observed inner-sphere, bidentate bridging and outer-sphere species kinetics congruent with the stepwise Eigen–Werner–Wilkins mechanism, in which a rapid formation of outer-sphere association precedes a slower conversion of outer-sphere to inner-sphere sulfate complexes. The rate limitation imposed by inner-sphere complex formation is likely linked to the displacement of protonated surface hydroxyl groups on the oxide surface by the adsorbing oxyanion. Outer-sphere complexes are responsible for rapid adsorption and desorption at I = 0.10 M seen in total sulfate, whereas inner-sphere species desorb more slowly. At I = 0.01 M, outer-sphere complexes similarly adsorb rapidly relative to inner-sphere complexes but desorb more slowly than inner-sphere complexes, possibly because of ionic strength effects on surface charge density. This work presents a novel, direct spectroscopic quantification of mixed surface species adsorption kinetics on a model mineral surface, which may be used to confirm proposed molecular mechanisms for other oxyanion adsorption to mineral surfaces. Our results enhance molecular-level understanding of oxyanion adsorption on soils and help predict their behavior in soils.
中文翻译:

内外球形氧合络合物动力学的光谱定量:离子强度和背景阳离子对硫酸盐吸附赤铁矿的影响
硫酸盐以内球和外球两种方式吸附在铁氧化物上。但是,并存的表面物质的时间依赖性尚不清楚。使用原位衰减全反射-傅立叶变换红外光谱,峰拟合和多元曲线分辨率分析,我们定量了两种离子强度(I = 0.01和0.10 M)下赤铁矿内和外球硫酸盐物种在赤铁矿上的吸附和解吸动力学。和背景阳离子(K +和Ca 2+)在pH 4.5下。我们通过实验观察到内球,双齿桥接和外球物种动力学与逐步的本征-韦纳-威尔金斯机理一致,其中快速形成外球缔合先于外球向内球硫酸盐配合物的缓慢转化。内球络合物形成所施加的速率限制很可能与吸附的氧阴离子取代了质子化表面羟基在氧化物表面上的位移有关。在总硫酸盐中,在I = 0.10 M时,外层络合物负责快速吸附和解吸,而内层物质的解吸更慢。在我= 0.01 M,外球络合物相对于内球络合物的吸附速度类似,但比内球络合物的吸附速度慢,这可能是由于离子强度对表面电荷密度的影响。这项工作提出了一种新颖的直接光谱定量模型矿物表面上混合表面物种吸附动力学的定量方法,可用于确定提出的其他氧阴离子吸附到矿物表面的分子机制。我们的研究结果增强了对氧阴离子在土壤上吸附的分子水平的了解,并有助于预测它们在土壤中的行为。
更新日期:2020-10-16
中文翻译:

内外球形氧合络合物动力学的光谱定量:离子强度和背景阳离子对硫酸盐吸附赤铁矿的影响
硫酸盐以内球和外球两种方式吸附在铁氧化物上。但是,并存的表面物质的时间依赖性尚不清楚。使用原位衰减全反射-傅立叶变换红外光谱,峰拟合和多元曲线分辨率分析,我们定量了两种离子强度(I = 0.01和0.10 M)下赤铁矿内和外球硫酸盐物种在赤铁矿上的吸附和解吸动力学。和背景阳离子(K +和Ca 2+)在pH 4.5下。我们通过实验观察到内球,双齿桥接和外球物种动力学与逐步的本征-韦纳-威尔金斯机理一致,其中快速形成外球缔合先于外球向内球硫酸盐配合物的缓慢转化。内球络合物形成所施加的速率限制很可能与吸附的氧阴离子取代了质子化表面羟基在氧化物表面上的位移有关。在总硫酸盐中,在I = 0.10 M时,外层络合物负责快速吸附和解吸,而内层物质的解吸更慢。在我= 0.01 M,外球络合物相对于内球络合物的吸附速度类似,但比内球络合物的吸附速度慢,这可能是由于离子强度对表面电荷密度的影响。这项工作提出了一种新颖的直接光谱定量模型矿物表面上混合表面物种吸附动力学的定量方法,可用于确定提出的其他氧阴离子吸附到矿物表面的分子机制。我们的研究结果增强了对氧阴离子在土壤上吸附的分子水平的了解,并有助于预测它们在土壤中的行为。


























 京公网安备 11010802027423号
京公网安备 11010802027423号