当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Influence of additive on the aggregation behavior of drug and cationic hydrotrope aniline hydrochloride mixtures: a physicochemical assessment
Journal of Physical Organic Chemistry ( IF 1.8 ) Pub Date : 2020-08-28 , DOI: 10.1002/poc.4120 Yousef G. Alghamdi 1 , Malik Abdul Rub 1 , Naved Azum 1, 2 , Abdullah M. Asiri 1, 2
Journal of Physical Organic Chemistry ( IF 1.8 ) Pub Date : 2020-08-28 , DOI: 10.1002/poc.4120 Yousef G. Alghamdi 1 , Malik Abdul Rub 1 , Naved Azum 1, 2 , Abdullah M. Asiri 1, 2
Affiliation
|
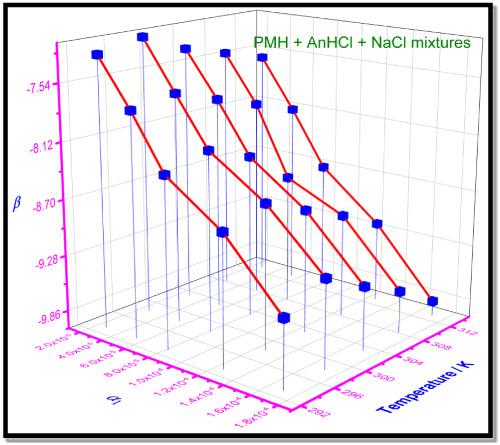
We investigated the influence of an additive (50 mmol kg–1 NaCl/300 mmol kg–1 urea [U]) on the aggregation of amphiphilic promethazine hydrochloride (PMH) drug and cationic hydrotrope–aniline hydrochloride (AnHCl) mixtures at various temperatures and ratios. The drug PMH is mostly used for the treatment of allergic symptoms. The obtained critical micelle concentration (cmc) values are well below the cmcid (ideal cmc) value, which confirms interactions between components PMH and AnHCl in the solution mixture. The micellar mole fraction (X1Rub, X1Rod, and X1id) of the first component (AnHCl) was evaluated using different models, which suggested greater involvement of AnHCl in mixed micelles (as expected); besides, the obtained micellar mole fraction values increase with increase in the mole fraction (α1) of AnHCl. The interaction parameter (β) values obtained were negative, which confirms attractive interaction or synergism among the components. Activity coefficients (
 [AnHCl] and
[AnHCl] and  [PMH]) values are always found below one confirming the nonideality as well as attractive interaction between the components. Different physicochemical parameters evaluated suggested attractive interaction between the components (PMH and AnHCl) in all studied solvents; however, in salt media, the interaction increases to some extent, whereas in urea media, it decreases to some extent. Diverse thermodynamic parameters (Gibbs free energy [ΔGm0], enthalpy [ΔHm0], and entropy [ΔSm0]) of micellization were computed and discussed separately. The excess free energy (
[PMH]) values are always found below one confirming the nonideality as well as attractive interaction between the components. Different physicochemical parameters evaluated suggested attractive interaction between the components (PMH and AnHCl) in all studied solvents; however, in salt media, the interaction increases to some extent, whereas in urea media, it decreases to some extent. Diverse thermodynamic parameters (Gibbs free energy [ΔGm0], enthalpy [ΔHm0], and entropy [ΔSm0]) of micellization were computed and discussed separately. The excess free energy (
 ) values of all systems were negative, suggesting higher stability of formed mixed micelles as compared with singular‐component micelles.
) values of all systems were negative, suggesting higher stability of formed mixed micelles as compared with singular‐component micelles.
中文翻译:

添加剂对药物和阳离子水溶助长剂苯胺盐酸盐混合物的聚集行为的影响:理化评估
我们研究了添加剂(50 mmol kg –1 NaCl / 300 mmol kg –1尿素[U])在不同温度和温度下对两亲性异丙嗪盐酸盐(PMH)药物和阳离子亲水性-苯胺盐酸盐(AnHCl)混合物聚集的影响比率。PMH药物主要用于治疗过敏症状。所获得的临界胶束浓度(cmc)值远低于cmc id(理想cmc)值,这证实了溶液混合物中组分PMH和AnHCl之间的相互作用。胶束摩尔分数(X 1 Rub,X 1 Rod和X 1使用不同的模型评估了第一组分(AnHCl)的id),这表明AnHCl在混合胶束中的参与程度更大(如预期);此外,所获得的胶束摩尔分数值与摩尔分数(增加而增加α 1 AnHCl的)。获得的相互作用参数(β)值为负,这证实了各组分之间有吸引力的相互作用或协同作用。活度系数( [AnHCl]和
[AnHCl]和 [PMH])值始终位于以下值,这表明组件之间存在非理想性以及有吸引力的相互作用。评估的不同理化参数表明,在所有研究的溶剂中,组分(PMH和AnHCl)之间都具有吸引作用。但是,在盐介质中,相互作用增加了一定程度,而在尿素介质中,相互作用减少了一定程度。计算并讨论了胶束化的不同热力学参数(吉布斯自由能[ ΔG m 0 ],焓[ ΔH m 0 ]和熵[ ΔS m 0 ])。多余的自由能(
[PMH])值始终位于以下值,这表明组件之间存在非理想性以及有吸引力的相互作用。评估的不同理化参数表明,在所有研究的溶剂中,组分(PMH和AnHCl)之间都具有吸引作用。但是,在盐介质中,相互作用增加了一定程度,而在尿素介质中,相互作用减少了一定程度。计算并讨论了胶束化的不同热力学参数(吉布斯自由能[ ΔG m 0 ],焓[ ΔH m 0 ]和熵[ ΔS m 0 ])。多余的自由能(
 )所有系统的值均为负值,表明与单组分胶束相比,形成的混合胶束具有更高的稳定性。
)所有系统的值均为负值,表明与单组分胶束相比,形成的混合胶束具有更高的稳定性。
更新日期:2020-08-28
 [AnHCl] and
[AnHCl] and  [PMH]) values are always found below one confirming the nonideality as well as attractive interaction between the components. Different physicochemical parameters evaluated suggested attractive interaction between the components (PMH and AnHCl) in all studied solvents; however, in salt media, the interaction increases to some extent, whereas in urea media, it decreases to some extent. Diverse thermodynamic parameters (Gibbs free energy [ΔGm0], enthalpy [ΔHm0], and entropy [ΔSm0]) of micellization were computed and discussed separately. The excess free energy (
[PMH]) values are always found below one confirming the nonideality as well as attractive interaction between the components. Different physicochemical parameters evaluated suggested attractive interaction between the components (PMH and AnHCl) in all studied solvents; however, in salt media, the interaction increases to some extent, whereas in urea media, it decreases to some extent. Diverse thermodynamic parameters (Gibbs free energy [ΔGm0], enthalpy [ΔHm0], and entropy [ΔSm0]) of micellization were computed and discussed separately. The excess free energy (
 ) values of all systems were negative, suggesting higher stability of formed mixed micelles as compared with singular‐component micelles.
) values of all systems were negative, suggesting higher stability of formed mixed micelles as compared with singular‐component micelles.
中文翻译:

添加剂对药物和阳离子水溶助长剂苯胺盐酸盐混合物的聚集行为的影响:理化评估
我们研究了添加剂(50 mmol kg –1 NaCl / 300 mmol kg –1尿素[U])在不同温度和温度下对两亲性异丙嗪盐酸盐(PMH)药物和阳离子亲水性-苯胺盐酸盐(AnHCl)混合物聚集的影响比率。PMH药物主要用于治疗过敏症状。所获得的临界胶束浓度(cmc)值远低于cmc id(理想cmc)值,这证实了溶液混合物中组分PMH和AnHCl之间的相互作用。胶束摩尔分数(X 1 Rub,X 1 Rod和X 1使用不同的模型评估了第一组分(AnHCl)的id),这表明AnHCl在混合胶束中的参与程度更大(如预期);此外,所获得的胶束摩尔分数值与摩尔分数(增加而增加α 1 AnHCl的)。获得的相互作用参数(β)值为负,这证实了各组分之间有吸引力的相互作用或协同作用。活度系数(
 [AnHCl]和
[AnHCl]和 [PMH])值始终位于以下值,这表明组件之间存在非理想性以及有吸引力的相互作用。评估的不同理化参数表明,在所有研究的溶剂中,组分(PMH和AnHCl)之间都具有吸引作用。但是,在盐介质中,相互作用增加了一定程度,而在尿素介质中,相互作用减少了一定程度。计算并讨论了胶束化的不同热力学参数(吉布斯自由能[ ΔG m 0 ],焓[ ΔH m 0 ]和熵[ ΔS m 0 ])。多余的自由能(
[PMH])值始终位于以下值,这表明组件之间存在非理想性以及有吸引力的相互作用。评估的不同理化参数表明,在所有研究的溶剂中,组分(PMH和AnHCl)之间都具有吸引作用。但是,在盐介质中,相互作用增加了一定程度,而在尿素介质中,相互作用减少了一定程度。计算并讨论了胶束化的不同热力学参数(吉布斯自由能[ ΔG m 0 ],焓[ ΔH m 0 ]和熵[ ΔS m 0 ])。多余的自由能(
 )所有系统的值均为负值,表明与单组分胶束相比,形成的混合胶束具有更高的稳定性。
)所有系统的值均为负值,表明与单组分胶束相比,形成的混合胶束具有更高的稳定性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号