当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
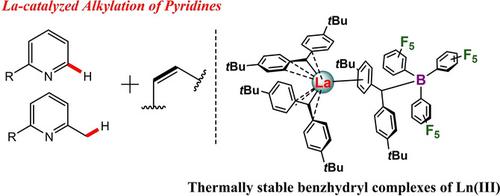
Tris(benzhydryl) and Cationic Bis(benzhydryl) Ln(III) Complexes: Exceptional Thermostability and Catalytic Activity in Olefin Hydroarylation and Hydrobenzylation with Substituted Pyridines
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-08-27 , DOI: 10.1002/adsc.202000782 Alexander N. Selikhov 1, 2 , Egor N. Boronin 1 , Anton V. Cherkasov 1 , Georgy K. Fukin 1 , Andrey S. Shavyrin 1 , Alexander A. Trifonov 1, 2
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-08-27 , DOI: 10.1002/adsc.202000782 Alexander N. Selikhov 1, 2 , Egor N. Boronin 1 , Anton V. Cherkasov 1 , Georgy K. Fukin 1 , Andrey S. Shavyrin 1 , Alexander A. Trifonov 1, 2
Affiliation
|
A series of Ln(III) tris(benzhydryl) complexes [(p‐tBu‐C6H4)2CH]3Ln (Ln=La (1), Nd (2), Y (3)) were synthesized by the salt metathesis reactions of LnHal3(THF)3.5 (Ln=La, Nd, Hal=Cl; Ln=Y, Hal=I) and [(p‐tBu‐C6H4)2CH]Na. In 1–3 the benzhydryl ligands are linked with the metal centres in η4‐coordination mode. For diamagnetic complexes 1 and 3 a fluxional behaviour was detected in solution. Complexes 1–3 proved to be thermally stable: no decomposition was observed even after heating their solutions in toluene‐d8 at 140 °C during 72 h. The reactions of 1 and 2 with B(C6F5)3 allowed for the synthesis of base‐free cationic complexes [(p‐tBu‐C6H4)2CH]2Ln[(p‐tBu‐C6H4)2CHB(C6F5)3] (Ln=La (4), Nd (5)) which adopt the structure of a contact ion pair. Combinations of 1–3 and borane ((B(C6F5)3, [Me2NHPh][B(C6F5)4], [Ph3C][B(C6F5)4]) as well as 4 and 5 were found to be highly efficient, regio‐ and chemoselective catalysts for hydroarylation and hydrobenzylation of C=C bonds of a variety of substrates with substituted pyridines. These catalysts enable highly challenging transformations such as hydrobenzylation of 1,1‐disubstituted and internal C=C bonds.
中文翻译:

三(苯甲酰基)和阳离子双(苯甲酰基)Ln(III)配合物:烯烃加氢芳基化和吡啶取代的苄基化中出色的热稳定性和催化活性
一系列LN(III)的三(二苯甲基)配合物[(p -吨卜-C 6 H ^ 4)2 CH] 3 LN(Ln为La(上1),钕(2),Y(3))是由合成LnHal的盐复分解反应3(THF)3.5(Ln为镧,钕,哈尔=氯; Ln为Y,哈尔= I)和[(p -吨卜-C 6 H ^ 4)2 CH]的Na。在1-3的二苯甲基的配体与金属中心连接的η 4 -coordination模式。对于抗磁性配合物1和3在溶液中检测到fluxional行为。配合物1 - 3被证明是热稳定的:即使在甲苯-d加热它们的溶液后,没有观察到分解8 72小时期间在140℃下。的反应1和2与B(C 6 ˚F 5)3允许游离碱阳离子络合物的合成[(p -吨卜-C 6 H ^ 4)2 CH] 2 LN [(p -吨卜-C 6高4)2CHB(C 6 F 5)3 ](Ln = La(4),Nd(5))采用接触离子对的结构。组合1 - 3和硼烷((B(C 6 ˚F 5)3,[我2 NHPh基] [B(C 6 ˚F 5)4 ],[PH 3 C] [B(C 6 ˚F 5)4 ])以及4和5被发现是高效的区域和化学选择性催化剂,可用于各种具有取代吡啶的底物的C = C键进行氢芳基化和加氢苄基化。这些催化剂能够实现极富挑战性的转化,例如1,1-二取代和内部C = C键的加氢苄基化。
更新日期:2020-08-27
中文翻译:

三(苯甲酰基)和阳离子双(苯甲酰基)Ln(III)配合物:烯烃加氢芳基化和吡啶取代的苄基化中出色的热稳定性和催化活性
一系列LN(III)的三(二苯甲基)配合物[(p -吨卜-C 6 H ^ 4)2 CH] 3 LN(Ln为La(上1),钕(2),Y(3))是由合成LnHal的盐复分解反应3(THF)3.5(Ln为镧,钕,哈尔=氯; Ln为Y,哈尔= I)和[(p -吨卜-C 6 H ^ 4)2 CH]的Na。在1-3的二苯甲基的配体与金属中心连接的η 4 -coordination模式。对于抗磁性配合物1和3在溶液中检测到fluxional行为。配合物1 - 3被证明是热稳定的:即使在甲苯-d加热它们的溶液后,没有观察到分解8 72小时期间在140℃下。的反应1和2与B(C 6 ˚F 5)3允许游离碱阳离子络合物的合成[(p -吨卜-C 6 H ^ 4)2 CH] 2 LN [(p -吨卜-C 6高4)2CHB(C 6 F 5)3 ](Ln = La(4),Nd(5))采用接触离子对的结构。组合1 - 3和硼烷((B(C 6 ˚F 5)3,[我2 NHPh基] [B(C 6 ˚F 5)4 ],[PH 3 C] [B(C 6 ˚F 5)4 ])以及4和5被发现是高效的区域和化学选择性催化剂,可用于各种具有取代吡啶的底物的C = C键进行氢芳基化和加氢苄基化。这些催化剂能够实现极富挑战性的转化,例如1,1-二取代和内部C = C键的加氢苄基化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号