Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solvatochromism and Excited-State Characteristics of Fluorenone-1-Carboxylic Acid
Journal of Luminescence ( IF 3.6 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.jlumin.2020.117619 Alvaro A. Montoya , Gregory M. Trout , Brian W. Williams , Swarna Basu
Journal of Luminescence ( IF 3.6 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.jlumin.2020.117619 Alvaro A. Montoya , Gregory M. Trout , Brian W. Williams , Swarna Basu
|
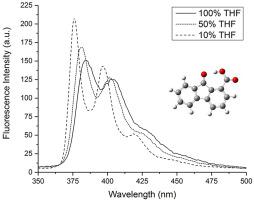
Abstract The solvatochromism of the fluorenone derivative 9-fluorenone-1-carboxylic acid (9F1C) was examined in binary cyclohexane-tetrahydrofuran mixtures, along with a theoretical investigation of possible excited state decay pathways. This fluorenone derivative differs from many previously investigated derivatives in that it possesses an electron withdrawing substituent and possibly undergoes intramolecular hydrogen bonding and proton transfer. A conventional Lippert-Mataga analysis was applied to the measured steady-state absorption and emission spectra to estimate the dipole moment difference between ground and emitting excited states. The estimated difference of ~2.8 D was found to be slightly larger than the average value of previously estimated differences for unsubstituted fluorenone (~2.4 D). The 9F1C derivative also differed from unsubstituted fluorenone in that fluorescence emission decreased, rather than increased, with increasing solvent polarity. Interpreting the possible cause of this difference prompted time-dependent density functional theory (TD-DFT) calculations of the relative energies of non-hydrogen bonded and intra-molecular hydrogen bonded excited states. These calculations suggested that an explanation for the decrease in emission for non-hydrogen bonded species could be enhancement of an intersystem-crossing (ISC) pathway. However, these same calculations suggested this explanation could not account for possible decreased emission from intra-molecular hydrogen-bonded species.
中文翻译:

1-芴酮-1-羧酸的溶剂致变色和激发态特性
摘要 在二元环己烷-四氢呋喃混合物中研究了芴酮衍生物 9-芴酮-1-羧酸 (9F1C) 的溶剂化显色,同时对可能的激发态衰变途径进行了理论研究。这种芴酮衍生物与许多先前研究的衍生物不同,它具有吸电子取代基,并且可能发生分子内氢键和质子转移。将传统的 Lippert-Mataga 分析应用于测量的稳态吸收和发射光谱,以估计基态和发射激发态之间的偶极矩差异。发现约 2.8 D 的估计差异略大于先前估计的未取代芴酮 (~2.4 D) 差异的平均值。9F1C 衍生物与未取代芴酮的不同之处还在于,随着溶剂极性的增加,荧光发射减少而不是增加。解释这种差异的可能原因促使时间相关密度泛函理论 (TD-DFT) 计算非氢键合和分子内氢键合激发态的相对能量。这些计算表明,非氢键物种排放减少的解释可能是系统间交叉 (ISC) 途径的增强。然而,这些相同的计算表明这种解释不能解释分子内氢键物种可能减少的发射。解释这种差异的可能原因促使时间相关密度泛函理论 (TD-DFT) 计算非氢键合和分子内氢键合激发态的相对能量。这些计算表明,非氢键物种排放减少的解释可能是系统间交叉 (ISC) 途径的增强。然而,这些相同的计算表明这种解释不能解释分子内氢键物种可能减少的发射。解释这种差异的可能原因促使时间相关密度泛函理论 (TD-DFT) 计算非氢键合和分子内氢键合激发态的相对能量。这些计算表明,非氢键物种排放减少的解释可能是系统间交叉 (ISC) 途径的增强。然而,这些相同的计算表明这种解释不能解释分子内氢键物种可能减少的发射。这些计算表明,非氢键物种排放减少的解释可能是系统间交叉 (ISC) 途径的增强。然而,这些相同的计算表明这种解释不能解释分子内氢键物种可能减少的发射。这些计算表明,非氢键物种排放减少的解释可能是系统间交叉 (ISC) 途径的增强。然而,这些相同的计算表明这种解释不能解释分子内氢键物种可能减少的发射。
更新日期:2020-12-01
中文翻译:

1-芴酮-1-羧酸的溶剂致变色和激发态特性
摘要 在二元环己烷-四氢呋喃混合物中研究了芴酮衍生物 9-芴酮-1-羧酸 (9F1C) 的溶剂化显色,同时对可能的激发态衰变途径进行了理论研究。这种芴酮衍生物与许多先前研究的衍生物不同,它具有吸电子取代基,并且可能发生分子内氢键和质子转移。将传统的 Lippert-Mataga 分析应用于测量的稳态吸收和发射光谱,以估计基态和发射激发态之间的偶极矩差异。发现约 2.8 D 的估计差异略大于先前估计的未取代芴酮 (~2.4 D) 差异的平均值。9F1C 衍生物与未取代芴酮的不同之处还在于,随着溶剂极性的增加,荧光发射减少而不是增加。解释这种差异的可能原因促使时间相关密度泛函理论 (TD-DFT) 计算非氢键合和分子内氢键合激发态的相对能量。这些计算表明,非氢键物种排放减少的解释可能是系统间交叉 (ISC) 途径的增强。然而,这些相同的计算表明这种解释不能解释分子内氢键物种可能减少的发射。解释这种差异的可能原因促使时间相关密度泛函理论 (TD-DFT) 计算非氢键合和分子内氢键合激发态的相对能量。这些计算表明,非氢键物种排放减少的解释可能是系统间交叉 (ISC) 途径的增强。然而,这些相同的计算表明这种解释不能解释分子内氢键物种可能减少的发射。解释这种差异的可能原因促使时间相关密度泛函理论 (TD-DFT) 计算非氢键合和分子内氢键合激发态的相对能量。这些计算表明,非氢键物种排放减少的解释可能是系统间交叉 (ISC) 途径的增强。然而,这些相同的计算表明这种解释不能解释分子内氢键物种可能减少的发射。这些计算表明,非氢键物种排放减少的解释可能是系统间交叉 (ISC) 途径的增强。然而,这些相同的计算表明这种解释不能解释分子内氢键物种可能减少的发射。这些计算表明,非氢键物种排放减少的解释可能是系统间交叉 (ISC) 途径的增强。然而,这些相同的计算表明这种解释不能解释分子内氢键物种可能减少的发射。


























 京公网安备 11010802027423号
京公网安备 11010802027423号