Cell ( IF 64.5 ) Pub Date : 2020-08-28 , DOI: 10.1016/j.cell.2020.08.015 Xin Jiang 1 , Yafei Yuan 2 , Jian Huang 3 , Shuo Zhang 1 , Shuchen Luo 3 , Nan Wang 1 , Debing Pu 4 , Na Zhao 5 , Qingxuan Tang 4 , Kunio Hirata 6 , Xikang Yang 3 , Yaqing Jiao 5 , Tomoyo Sakata-Kato 5 , Jia-Wei Wu 7 , Chuangye Yan 1 , Nobutaka Kato 5 , Hang Yin 4 , Nieng Yan 1
|
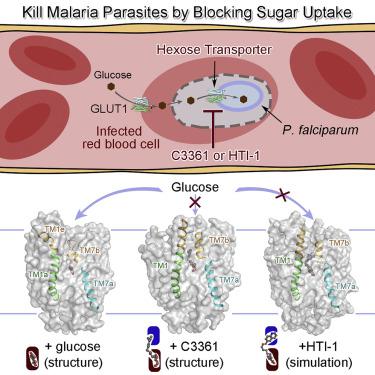
Plasmodium species, the causative agent of malaria, rely on glucose for energy supply during blood stage. Inhibition of glucose uptake thus represents a potential strategy for the development of antimalarial drugs. Here, we present the crystal structures of PfHT1, the sole hexose transporter in the genome of Plasmodium species, at resolutions of 2.6 Å in complex with D-glucose and 3.7 Å with a moderately selective inhibitor, C3361. Although both structures exhibit occluded conformations, binding of C3361 induces marked rearrangements that result in an additional pocket. This inhibitor-binding-induced pocket presents an opportunity for the rational design of PfHT1-specific inhibitors. Among our designed C3361 derivatives, several exhibited improved inhibition of PfHT1 and cellular potency against P. falciparum, with excellent selectivity to human GLUT1. These findings serve as a proof of concept for the development of the next-generation antimalarial chemotherapeutics by simultaneously targeting the orthosteric and allosteric sites of PfHT1.
中文翻译:

阻止糖摄入疟疾寄生虫恶性疟原虫的结构基础。
疟原虫是疟疾的病原体,在血液阶段依靠葡萄糖提供能量。因此,抑制葡萄糖摄取代表了抗疟药开发的潜在策略。在这里,我们介绍了疟原虫物种基因组中唯一的己糖转运蛋白PfHT1的晶体结构,与D-葡萄糖复合时的分辨率为2.6,与中度选择性抑制剂C3361的分辨率为3.7。尽管两个结构都显示出封闭的构象,但C3361的结合会引起明显的重排,从而导致额外的口袋。该抑制剂结合诱导的口袋为PfHT1特异性抑制剂的合理设计提供了机会。在我们设计的C3361衍生物中,有一些表现出对PfHT1的抑制作用增强以及对恶性疟原虫,对人GLUT1具有优异的选择性。这些发现通过同时靶向PfHT1的正构和变构位点,为下一代抗疟化学疗法的发展提供了概念证明。


























 京公网安备 11010802027423号
京公网安备 11010802027423号